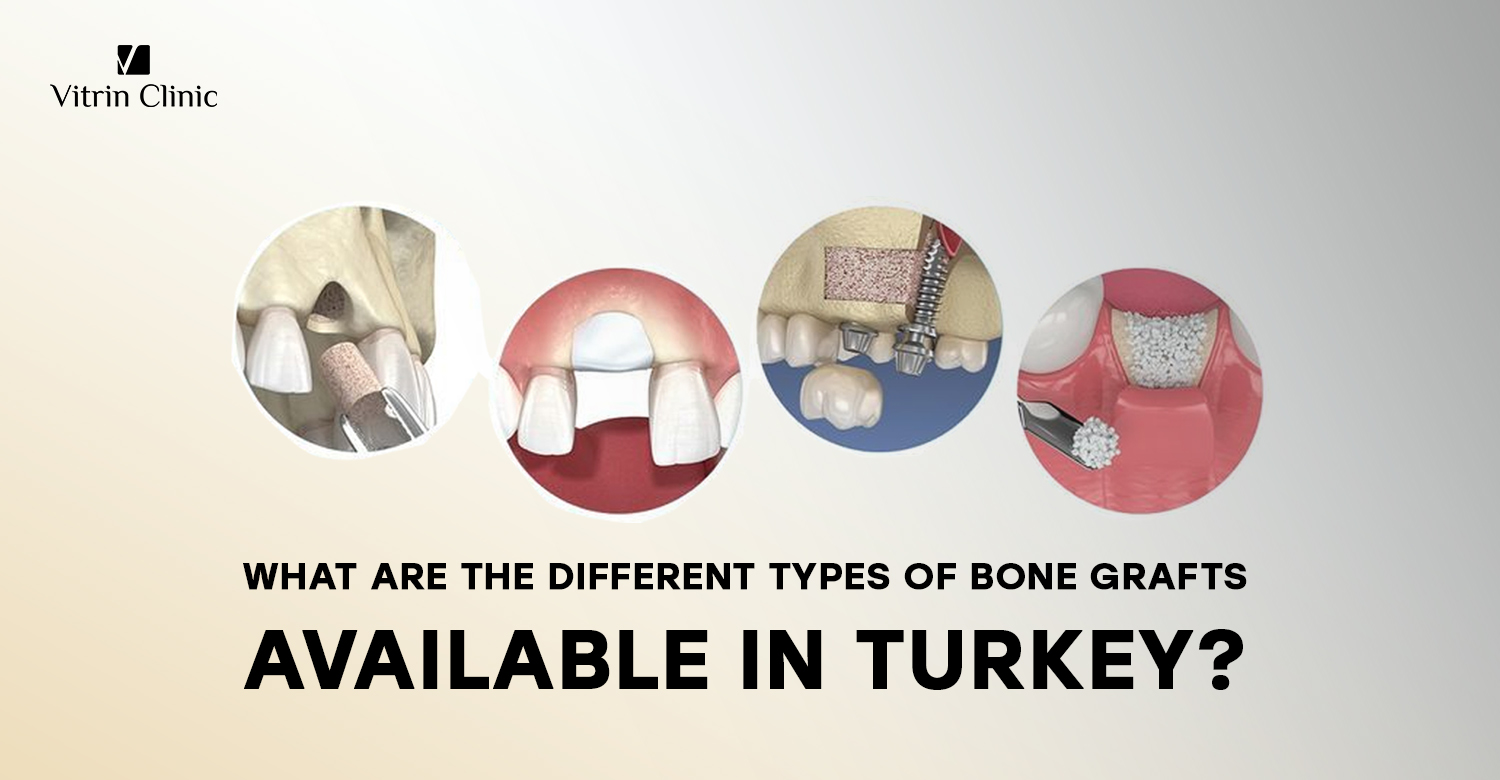
ما هي الأنواع المختلفة من ترقيع العظام المستخدمة في إجراءات طب الأسنان؟
في إجراءات طب الأسنان، تعتبر ترقيعات العظام ضرورية لإعادة بناء هيكل العظم، خاصة لدعم الزرعات. الأنواع الأربعة الأساسية هي الترقيع الذاتي، والترقيع من متبرعين، والترقيع الحيواني، والترقيع الصناعي. يستخدم الترقيع الذاتي عظمًا مأخوذًا من جسم المريض نفسه، مما يوفر معدلات نجاح عالية.
يأتي الترقيع من متبرعين بشريين، عادة من جثث، ويتم معالجته لضمان السلامة. أما الترقيع الحيواني فيُستخلص من مصادر حيوانية، غالبًا من الأبقار.
الترقيع الصناعي مصنوع من مواد متوافقة حيويًا. كل نوع له فوائد ومخاطر ومؤشرات استخدام مميزة. يعتمد الاختيار على تشريح المريض وتاريخه الطبي والمتطلبات السريرية الخاصة للإجراء الذي يتم تنفيذه.
ما هو ترقيع العظام ولماذا توجد أنواع مختلفة منه؟
ترقيع العظام هو إجراء جراحي يُستخدم لاستبدال أو زيادة العظام المفقودة، خصوصًا في التحضير لزرعات الأسنان أو لعلاج أمراض اللثة. توجد أنواع مختلفة من الترقيعات لأن حالة كل مريض وقدرته على الالتئام تختلف. بعض المرضى يحتاجون إلى شفاء أسرع وتكامل أفضل، مما يجعل الترقيع الذاتي الخيار المثالي.
أما الآخرون الذين لا يملكون كمية كافية من العظام المانحة فقد يكون الترقيع من متبرعين أو الترقيع الصناعي أكثر ملاءمة. عوامل مثل خطر العدوى، ومدة الشفاء، والتكلفة، والمخاوف الأخلاقية أو الدينية قد تؤثر أيضًا على الاختيار. في النهاية، توفر الخيارات المتعددة للأطباء القدرة على تخصيص خطة العلاج لتحقيق النجاح الوظيفي والجمالي.
كيف يعمل ترقيع العظام في زراعة الأسنان؟
في زراعة الأسنان، يُستخدم ترقيع العظام عندما يفتقر المريض إلى كمية كافية من عظم الفك لدعم الزرعة بثبات. بعد تقييم حجم العظم باستخدام الصور الطبية، يختار الطبيب نوع الترقيع المناسب ويضعه في المنطقة المحتاجة.
بمرور الوقت، يعمل هذا الترقيع كهيكل داعم يسمح لخلايا العظام الطبيعية بالنمو والاندماج مع المادة. بعد الشفاء الكامل، والذي قد يستغرق عدة أشهر، يمكن للعظم الجديد أن يدعم الزرعة السنية بشكل آمن. هذه العملية ضرورية لاستقرار الزرعة على المدى الطويل، خاصة في المناطق التي حدث فيها فقدان للأسنان أو أمراض اللثة.
ماذا يحدث أثناء عملية تجدد العظام؟
تبدأ عملية تجدد العظام بعد وضع الترقيع، عندما يستجيب الجسم بإرسال الخلايا إلى الموقع. في البداية، تتكون الجلطات الدموية، ثم تهاجر الخلايا البانية للعظم (الخلايا المكونة للعظام) لتبدأ بتكوين عظم جديد. بمرور الوقت، يتم امتصاص مادة الترقيع تدريجيًا واستبدالها بعظم طبيعي. تُعرف هذه العملية بالاندماج العظمي.
تعتمد سرعة وكفاءة هذه العملية على نوع الترقيع، وتروية الموقع بالدم، وصحة المريض العامة. والنتيجة المثالية هي عظم قوي وطبيعي قادر على دعم زرعات الأسنان أو التركيبات الأخرى، مما يعيد الشكل والوظيفة للفك.
ما العوامل التي تحدد نسب نجاح ترقيع العظام؟
هناك عدة عوامل تؤثر على نجاح ترقيع العظام، منها الحالة الصحية العامة للمريض، وخاصة الأمراض مثل السكري أو التدخين الذي يضعف الالتئام. كما أن نوع وجودة مادة الترقيع لهما تأثير كبير — فالترقيع الذاتي عادة ما يحقق معدلات نجاح أعلى بفضل خصائصه المحفزة لنمو العظم.
تُعد التقنية الجراحية السليمة، وتوفر تدفق دم كافٍ في موقع الترقيع، والرعاية بعد الجراحة أمورًا حاسمة أيضًا. بالإضافة إلى ذلك، فإن حجم العيب العظمي ووجود العدوى يمكن أن يؤثرا في النتائج. عندما تتكامل كل العوامل إيجابيًا، يندمج الترقيع جيدًا ويوفر قاعدة مستقرة للترميمات السنية.
كم من الوقت يستغرق اندماج العظام عادة؟
عادة ما يستغرق اندماج العظام، أو ما يُعرف بالاندماج العظمي، ما بين 3 إلى 6 أشهر، وذلك حسب نوع الترقيع وصحة المريض وموقعه. يندمج الترقيع الذاتي بسرعة أكبر لاحتوائه على خلايا حية، بينما قد تستغرق الترقيعات الصناعية أو الحيوانية وقتًا أطول.
تلتئم الترقيعات الصغيرة في المناطق الغنية بالأوعية الدموية أسرع من تلك الكبيرة أو الفقيرة بالتروية. عوامل مثل التدخين أو الأمراض المزمنة أو سوء نظافة الفم قد تؤخر الاندماج. سيراقب الطبيب تقدم الشفاء من خلال الصور، وعند التأكد من الاندماج الكافي يمكن تجهيز الموقع للزرعة أو الترميم النهائي.
ما الفئات الرئيسية لمواد ترقيع العظام؟
تصنف مواد ترقيع العظام إلى ترقيع ذاتي، وترقيع من متبرعين، وترقيع حيواني، وترقيع صناعي.
- الترقيع الذاتي يأتي من جسم المريض نفسه ويحتوي على خلايا عظمية حية، مما يعزز الشفاء الممتاز.
- الترقيع من المتبرعين مصدره أنسجة بشرية مانحة، ويوفر دعمًا هيكليًا جيدًا مع قدرة شفاء أقل.
- الترقيع الحيواني مستخلص من الحيوانات (عادة من الأبقار) ويعمل كهيكل داعم لتكوين العظم الجديد.
- الترقيع الصناعي مصنوع من مواد متوافقة حيويًا مصممة لتحفيز تجدد العظام.
كل فئة تقدم مزايا مختلفة من حيث الشفاء والتوافر والتكلفة. يختار الأطباء المادة المناسبة بناءً على صحة المريض وحجم الترقيع وأهداف الإجراء.
كيف يتم تصنيف ترقيعات العظام طبيًا؟
يتم تصنيف ترقيعات العظام طبيًا بناءً على مصدرها وخصائصها البيولوجية.
- الترقيع الذاتي مأخوذ من المريض نفسه.
- الترقيع من المتبرعين هو نسيج بشري مانح.
- الترقيع الحيواني مأخوذ من نوع آخر.
- الترقيع الصناعي مصنوع من مواد اصطناعية.
بيولوجيًا، تُصنف أيضًا إلى محفزة لتكوين العظم (تحفز تكوين العظم)، أو موصلة للعظم (تعمل كهيكل داعم)، أو محفزة للخلايا البانية للعظم. الترقيع الذاتي هو النوع الوحيد الذي يمتلك الخصائص الثلاث جميعها بشكل طبيعي. يساعد فهم هذه التصنيفات الأطباء على توقع كيفية تصرف الترقيع واندماجه في جسم المريض، لضمان أفضل تطابق لكل حالة جراحية.
ما الذي يحدد الاختيار بين أنواع الترقيع المختلفة؟
يعتمد القرار بين أنواع الترقيع على عدة عوامل: صحة المريض، حجم العيب العظمي، مدى استعجال الإجراء، وخطر المضاعفات. غالبًا ما يُفضل الترقيع الذاتي لقدرته الممتازة على الاندماج، لكنه يتطلب موقعًا جراحيًا ثانيًا.
يوفر الترقيع من المتبرعين دعمًا جيدًا دون الحاجة لموقع ثانٍ لكنه قد يندمج بشكل أبطأ. الترقيع الحيواني متوفر على نطاق واسع وفعال في العديد من الحالات لكنه يتحلل ببطء. أما الترقيع الصناعي فهو مثالي للمرضى الذين لديهم اعتبارات أخلاقية أو دينية. كما يأخذ الجراح بعين الاعتبار التكلفة وخطر العدوى والهدف من الإجراء سواء كان دعمًا هيكليًا أو زيادة في الحجم أو كليهما.
ما هو الترقيع الذاتي وكيف يعمل؟
الترقيع الذاتي هو ترقيع عظمي مأخوذ من جسم المريض نفسه. ويُعتبر الأكثر توافقًا وفعالية من الناحية البيولوجية لأنه يحتوي على خلايا عظمية حية وعوامل نمو طبيعية. عند زراعته، يعزز الترقيع الذاتي شفاء العظم من خلال المساهمة المباشرة بخلايا تكوين العظم ودعم تجدد العظم بشكل طبيعي.
تشمل المواقع الشائعة لأخذ العظم الذاتي منطقة الذقن أو الفرع الفكي أو مناطق خارج الفم مثل عظم الحوض. وبما أنه مأخوذ من المريض نفسه، فلا يوجد خطر من رفض مناعي أو انتقال أمراض، مما يجعله خيارًا موثوقًا جدًا في إعادة بناء الأسنان وزراعتها.
ما الذي يجعل الترقيع الذاتي المعيار الذهبي في ترقيع العظام؟
يُعتبر الترقيع الذاتي المعيار الذهبي في ترقيع العظام لأنه يحتوي على الخصائص الثلاث الأساسية لتجدد العظام: توليد العظم، ودعم العظم، وتحفيز تكوينه. هذا يعني أنه لا يعمل فقط كهيكل داعم، بل يحتوي أيضًا على خلايا حية وبروتينات طبيعية تحفز نمو العظام.
يندمج بسرعة وبشكل متوقع، مما يقلل من مضاعفات الشفاء ويزيد فرص النجاح، خاصة في زراعة الأسنان. بالإضافة إلى ذلك، نظرًا لأن المادة مأخوذة من جسم المريض نفسه، فلا يوجد خطر من الرفض المناعي أو انتقال الأمراض. هذه العوامل مجتمعة تجعل الترقيع الذاتي الأكثر فعالية وتفضيلًا في العديد من الحالات السريرية.
لماذا يحقق الترقيع الذاتي أعلى معدلات النجاح؟
يحقق الترقيع الذاتي أعلى معدلات النجاح بفضل توافقه البيولوجي وقدرته الطبيعية على التجدد. فهو يحتوي على خلايا عظمية حية (الخلايا البانية للعظم) وعوامل نمو تساهم مباشرة في تكوين عظم جديد. على عكس المواد المانحة أو الصناعية، يندمج الترقيع الذاتي بسلاسة دون تحفيز استجابات مناعية أو تأخير في الالتئام.
قدرته على تحفيز تكوين الأوعية الدموية وتجديد العظم بسرعة تمنحه ميزة سريرية لتحقيق نتائج قوية ودائمة. وعند جمعه وزراعته بشكل صحيح، يقلل الترقيع الذاتي من خطر فشل الترقيع، مما يجعله مثاليًا لتطبيقات حرجة مثل زراعة الأسنان ورفع الجيوب الأنفية وتكبير الحافة العظمية.
ما هي الخصائص المولدة للعظم في الترقيع الذاتي؟
الترقيع الذاتي فريد في امتلاكه خصائص مولدة للعظم، أي أنه يساهم مباشرة في تكوين عظم جديد من خلال نشاط الخلايا البانية للعظم الحية. وعلى عكس أنواع الترقيع الأخرى التي تكتفي بدعم أو تحفيز نمو العظم، فإن الترقيع الذاتي يُنتج العظم فعليًا. كما يحتوي على عوامل نمو تُسرع الالتئام وتحسن تكوين الأوعية الدموية.
هذا ما يجعل الطعوم الذاتية ذات قيمة خاصة في جراحات الأسنان التي تتطلب اندماجًا سريعًا وقويًا. طبيعتها المولدة للعظام تؤدي إلى شفاء أسرع، ومضاعفات أقل، ومعدلات نجاح أعلى لزراعة الأسنان، مما يعزز مكانتها كأكثر مواد ترقيع العظام فاعلية بيولوجية متاحة.
كيف تساعد الطعوم الذاتية في تعزيز شفاء العظام الطبيعي؟
تُحفِّز الطعوم الذاتية شفاء العظام الطبيعي من خلال إدخال خلايا العظام الحية وعوامل النمو الخاصة بالمريض في موقع الطعم. تبدأ هذه المكونات عملية تكوين العظام على الفور تقريبًا، مما يسمح باندماج سلس بين الطعم والعظم القائم.
تُنتج الخلايا المولدة للعظام نسيجًا عظميًا جديدًا، بينما تُحفّز البروتينات المحفزة للعظم الأنسجة المحيطة للمشاركة في عملية التجديد. تحاكي هذه العملية آليات ترميم العظام الطبيعية، مما يضمن اندماجًا أسرع وأكثر استقرارًا. وعلى عكس الأنواع الأخرى من الطعوم، لا تتطلب الطعوم الذاتية تكيف الجسم مع مادة غريبة، مما يقلل من المضاعفات ويُهيئ بيئة مثالية للشفاء الكامل والموثوق.
من أين تُؤخذ الطعوم الذاتية عادةً؟
تُستخلص الطعوم الذاتية عادةً من مواقع تبرع داخل الفم أو خارجه، اعتمادًا على حجم ونوع العظم المطلوب. تشمل المواقع داخل الفم منطقة الذقن (الارتفاق الفكي السفلي)، وفرع الفك السفلي، والحديبة الفكية العلوية، وهي توفر عظمًا مناسبًا للطعوم الصغيرة أو الموضعية.
أما في الحالات التي تتطلب كميات أكبر من العظم، فتُفضل المواقع خارج الفم مثل الحافة الحرقفية (الورك) أو الظنبوب، نظرًا لغناها بالعظم الإسفنجي. يعتمد اختيار موقع التبرع على سهولة الوصول، وصحة المريض، وكمية العظم المطلوبة. يسعى الجراحون لتقليل المضاعفات في موقع التبرع مع ضمان جودة عظم مثالية للطعوم.
ما هي تقنية أخذ الطعم من الارتفاق الفكي السفلي؟
يُعد الارتفاق الفكي السفلي (منطقة الذقن) موقعًا شائعًا داخل الفم لاستخلاص الطعوم العظمية الذاتية. تتضمن التقنية إجراء شق صغير داخل الشفة السفلية للوصول إلى العظم. يُزال جزء من العظم على شكل كتلة أو جسيمات صغيرة بعناية مع حماية البنى الحيوية مثل جذور الأسنان والأعصاب الذقنية.
يُشكَّل العظم المستخلص ويوضع في موقع الطعم المطلوب. تُوفر هذه المنطقة عظمًا قشريًا كثيفًا مثاليًا للاحتياجات الصغيرة إلى المتوسطة. تتيح التقنية سهولة الوصول مع ندوب خارجية طفيفة، رغم احتمال حدوث خدر مؤقت أو انزعاج بعد العملية.
كيف يتم أخذ العظم من فرع الفك السفلي؟
تتضمن عملية أخذ العظم من فرع الفك السفلي، الواقع خلف الأضراس، الوصول إلى الموقع عبر شق داخل الفم بالقرب من الجزء الخلفي للفك. يقوم الجراح بإزالة جزء من العظم بعناية مع تجنب إصابة العصب السنخي السفلي.
يُوفر فرع الفك السفلي عظمًا قشريًا عالي الجودة يتميز بالقوة والكثافة، مما يجعله مثاليًا للطعوم الداعمة للأسنان. تُستخدم هذه التقنية غالبًا في عمليات زيادة العظم الخلفي ودعم الزرعات. يُعد التورم أو الانزعاج الطفيف بعد الجراحة أمرًا شائعًا لكنه مؤقت عادةً. يوفر هذا الموقع مصدرًا موثوقًا للعظم مع ندوب أقل ومضاعفات طويلة الأمد محدودة.
ما هي مواقع الطعوم الذاتية خارج الفم؟
تشمل مواقع الطعوم الذاتية خارج الفم مناطق خارج تجويف الفم، مثل الحافة الحرقفية، والظنبوب، وأحيانًا قبة الجمجمة. تُوفر هذه المواقع كميات كبيرة من العظم الإسفنجي والقشري، مما يجعلها مثالية للعمليات الترميمية الكبرى مثل إعادة بناء الفك أو إصلاح الكسور.
يتطلب الاستخلاص من هذه المناطق تخديرًا عامًا ويُنشئ موقعًا جراحيًا إضافيًا، مما قد يزيد من الانزعاج ومدة الشفاء. ومع ذلك، فإن جودة وكمية العظم المتاحة غالبًا ما تبرر استخدامها، خاصة في جراحات الأسنان أو الوجه والفكين المعقدة التي تتطلب مادة ترقيع قوية.
أي من المواقع داخل الفم توفر أفضل جودة للعظم؟
يُعد فرع الفك السفلي والارتفاق الفكي السفلي أفضل المواقع داخل الفم من حيث جودة العظم بسبب تركيبهما القشري الكثيف الذي يوفر استقرارًا ممتازًا للطعم. تحتوي هذه المناطق على طعوم ذاتية تتميز بإمكانات توليد عظم قوية واندماج موثوق.
يمكن أيضًا استخدام الحديبة الفكية ومآخذ الخلع، لكن العظم فيها أكثر ليونة، لذا يُفضل استخدامها في الطعوم الجزيئية بدلًا من الدعم البنيوي. يعتمد اختيار الموقع الأفضل داخل الفم على حجم العظم المطلوب وسهولة الوصول والهياكل التشريحية المجاورة. تتميز هذه المواقع بسهولة الوصول وندوب طفيفة، مما يجعلها مناسبة للإجراءات السنية الموضعية.
ما هي مزايا وعيوب الطعوم الذاتية؟
تشمل مزايا الطعوم الذاتية التوافق البيولوجي الفريد، ومعدلات النجاح العالية، والقدرة الفعالة على تكوين العظام. تندمج بسرعة ولا تحمل خطر الرفض المناعي أو نقل العدوى. ومع ذلك، تشمل العيوب الحاجة إلى موقع جراحي ثانٍ، مما يزيد من وقت العملية واحتمال المضاعفات مثل الألم أو إصابة الأعصاب.
قد يحد حجم العظم المحدود أيضًا من استخدامها في العيوب الكبيرة. رغم هذه التحديات، فإن النتائج المتوقعة والفوائد التجديدية للطعوم الذاتية تجعلها الخيار المفضل في العديد من عمليات ترقيع العظام السنية، خصوصًا عندما تكون استقرار الزرعات على المدى الطويل أولوية.
ما هي الفوائد الرئيسية لاستخدام عظمك الخاص؟
يضمن استخدام عظمك في عمليات الترقيع أعلى درجات التوافق وإمكانات الشفاء. تحتوي الطعوم الذاتية على خلايا حية، وعوامل نمو، ودعامة طبيعية، مما يجعلها النوع الوحيد من الطعوم الذي يدعم جميع جوانب تجديد العظم.
نظرًا لأن النسيج مأخوذ من جسمك، فلا يوجد خطر من تفاعل مناعي أو نقل عدوى. يحدث الاندماج بسرعة، وتكون النتائج طويلة الأمد أكثر قابلية للتنبؤ. بالإضافة إلى ذلك، يقلل استخدام العظم الذاتي من الاعتماد على المواد المانحة أو البدائل الصناعية، مما يمنح المريض والطبيب تحكمًا أكبر في عملية الشفاء والنتائج النهائية.
ما هي المضاعفات التي قد تنشأ من إجراءات الطعوم الذاتية؟
على الرغم من فعاليتها، فإن إجراءات الطعوم الذاتية قد تحمل بعض المخاطر. أكثر المضاعفات شيوعًا هو مشاكل موقع التبرع، والتي قد تشمل الألم أو التورم أو العدوى أو تلفًا مؤقتًا في الأعصاب. يمكن أن يؤدي الاستخلاص من مناطق مثل الارتفاق الفكي أو فرع الفك إلى خدر أو تغير في الإحساس. في حالات نادرة، قد يتسبب الأسلوب الجراحي الخاطئ في تلف جذور الأسنان أو الأعصاب.
يزيد وجود موقع جراحي إضافي من مدة العملية وفترة التعافي. ومع ذلك، تكون معظم المضاعفات طفيفة وتزول مع الرعاية المناسبة بعد الجراحة. يجب إبلاغ المرضى بالمخاطر ومتابعتهم عن قرب أثناء التعافي.
كيف يؤثر أخذ العظم على شفاء موقع التبرع؟
يختلف شفاء موقع التبرع حسب الموقع وكمية العظم المأخوذة. عادةً ما تلتئم المواقع داخل الفم مثل فرع الفك أو الارتفاق الفكي خلال بضعة أسابيع، مع احتمال حدوث تورم أو كدمات أو تغير مؤقت في الإحساس.
قد تستغرق المواقع خارج الفم مثل الحافة الحرقفية وقتًا أطول للشفاء بسبب عمق الجراحة وحجم العظم المأخوذ. يُحسن الأسلوب الجراحي الدقيق، وتقليل الصدمات، والحفاظ على نظافة الفم النتائج بشكل كبير. تساعد العناية بعد العملية، بما في ذلك الراحة والمضادات الحيوية عند الحاجة، على تقليل خطر العدوى وتسريع الشفاء. تُعيد معظم مواقع التبرع توليد العظم بمرور الوقت دون تأثيرات طويلة الأمد تُذكر.
ما هي الطعوم المأخوذة من متبرعين (Allografts) ومتى يُنصح بها؟
الطعوم المأخوذة من متبرعين هي مواد عظمية مستخلصة من متبرعين بشريين (عادة من جثث)، تُعالج لضمان الأمان والتوافق الحيوي. تُستخدم عادة عندما لا يستطيع المريض تقديم كمية كافية من العظم الذاتي أو يرغب في تجنب موقع جراحي ثانٍ. يُنصح باستخدامها في رفع الحافة العظمية، ورفع الجيب الأنفي، وعلاج عيوب اللثة، وتحضير مواقع الزرع.
تعمل هذه الطعوم كدعامات ناقلة للعظم (osteoconductive)، تدعم تكوين عظم جديد من العظم الطبيعي المحيط. ورغم أنها ليست مولدة للعظم مثل الطعوم الذاتية، إلا أن توفرها وسهولة استخدامها ونتائجها المتوقعة تجعلها خيارًا موثوقًا في العديد من الحالات السريرية، خصوصًا عندما يكون المطلوب تجديدًا عظميًا معتدلًا دون زيادة معاناة المريض.
كيف تُعالج الطعوم المأخوذة من متبرعين وتُحضّر؟
تخضع الطعوم المأخوذة من متبرعين لمعالجة مكثفة لإزالة المكونات الخلوية مع الحفاظ على مصفوفة العظم التي تدعم التجديد. بعد فحص المتبرع، يُنظف العظم ويُعالج بمواد كيميائية لإزالة البروتينات والدهون ثم يُعقَّم.
يمكن تجفيف النسيج بالتجميد (التجفيف بالتجميد) لزيادة مدة صلاحيته أو إزالة المعادن منه لتعزيز خصائصه المحفزة للعظم. تقلل هذه العمليات خطر التفاعلات المناعية أو نقل العدوى مع الحفاظ على سلامة بنية العظم. النتيجة هي منتج آمن ومعقم يمكن استخدامه مباشرة في جراحات الأسنان. تختلف طرق المعالجة حسب الخصائص المطلوبة للطعم مثل الكثافة أو التركيب أو الشكل.
ما هي عملية التجفيف بالتجميد للطعوم المأخوذة من متبرعين؟
تُعرف عملية التجفيف بالتجميد أيضًا باسم “التحليل بالتجميد”، وهي تقنية تُزال فيها الرطوبة من العظم المأخوذ من المتبرع في ظروف منخفضة الحرارة وتحت تفريغ هوائي. تحافظ هذه الطريقة على مصفوفة العظم الهيكلية وتجعلها مستقرة في درجة حرارة الغرفة لفترات طويلة. يمكن ترطيب الطعوم المجففة بالتجميد بمحلول ملحي أو مضادات حيوية قبل الاستخدام.
تُقلل هذه الطريقة كذلك من احتمالية التلوث البكتيري أو الفيروسي مع الحفاظ على الخصائص الناقلة للعظم. تُعد هذه التقنية مفيدة جدًا لإنتاج مواد مثل FDBA (طعوم العظام المجففة بالتجميد) المستخدمة على نطاق واسع في جراحات الأسنان واللثة لسهولة تخزينها وفعاليتها.
كيف تُعقَّم الطعوم المأخوذة من متبرعين لمنع انتقال الأمراض؟
تُعقَّم الطعوم المأخوذة من متبرعين باستخدام طرق مثل الإشعاع الجامي، أو غاز أكسيد الإيثيلين، أو المعالجات الكيميائية للقضاء على الممرضات بما في ذلك البكتيريا والفيروسات والفطريات. تُطبق هذه التقنيات بعد تنظيف ومعالجة الأنسجة الأولية.
يُعد الإشعاع الجامي الطريقة الأكثر شيوعًا وفعالية، لكن يجب التحكم فيه بعناية للحفاظ على النشاط البيولوجي للطعم.
يُنفذ التعقيم وفقًا لإرشادات تنظيمية صارمة لضمان أمان النسيج دون التأثير على قوته الميكانيكية أو قدرته التجديدية. وبالاقتران مع فحوصات المتبرعين والاختبارات المصلية، تُعد هذه الخطوات ضمانًا لسلامة الطعوم في جراحات ترقيع العظام السنية الحديثة.
ما هي إجراءات مراقبة الجودة التي تضمن سلامة الطعوم المأخوذة من متبرعين؟
تُضمن سلامة الطعوم من خلال فحص دقيق للمتبرعين، واختبارات معملية، ومعايير صارمة لمعالجة الأنسجة. يُفحص المتبرعون بحثًا عن الأمراض المعدية، والتاريخ الطبي، وعوامل نمط الحياة التي قد تشكل خطرًا. بعد الاستخلاص، تُفحص الأنسجة للكشف عن فيروس نقص المناعة البشرية، والتهاب الكبد، والزهري، وغيرها من مسببات الأمراض.
تلتزم مرافق المعالجة بلوائح إدارة الغذاء والدواء الأمريكية (FDA) وجمعية بنوك الأنسجة الأمريكية (AATB)، بما في ذلك بروتوكولات التعقيم، وأنظمة التتبع، وطرق التنظيف الموثقة. تضمن اختبارات الدُفعات والتوثيق الاتساق والسلامة في جميع الطعوم. تقلل هذه التدابير الرقابية من خطر نقل العدوى أو رفض الطعم، مما يجعلها خيارًا آمنًا وفعالًا في التطبيقات السنية السريرية.
ما هي أنواع الطعوم المأخوذة من متبرعين المتوفرة؟
تتوفر الطعوم المأخوذة من متبرعين بأشكال وتركيبات مختلفة، مما يوفر مرونة لمجموعة واسعة من الإجراءات السنية. الأنواع الأكثر شيوعًا تشمل:
- طعوم العظام المجففة بالتجميد (FDBA): تحتفظ بالمحتوى المعدني وتوفر دعمًا هيكليًا قويًا.
- مصفوفة العظم المنزوعة المعادن (DBM): تُعالج كيميائيًا لإزالة المعادن وكشف البروتينات التي تعزز التحفيز الذاتي.
- طعوم العظام المنزوعة المعادن والمجففة بالتجميد (DFDBA): تجمع بين مزايا إزالة المعادن وطول فترة التخزين.
قد تكون الطعوم أيضًا قشرية، أو إسفنجية، أو مزيجًا من كليهما، بحيث تخدم كل منها أغراضًا سريرية محددة وفقًا للحاجة إلى القوة أو سرعة إعادة البناء.
ما هي مصفوفة العظم المنزوعة المعادن (DBM)؟
مصفوفة العظم المنزوعة المعادن (DBM) هي طعم مأخوذ من متبرع أُزيل منه المحتوى المعدني غير العضوي، مما يترك مصفوفة غنية بالكولاجين تحتفظ بخصائصها الناقلة والمحفزة للعظم. تكشف عملية إزالة المعادن عن بروتينات النمو الطبيعية مثل بروتينات BMP التي تساعد على تحفيز خلايا تكوين العظم.
تُستخدم الـ DBM عادة في صورة جسيمات أو معجون وهي مثالية لملء العيوب العظمية الصغيرة ودعم شفاء العظام في إجراءات اللثة أو الزرعات. ورغم افتقارها إلى القوة الهيكلية، فإن قدرتها على تحفيز التجديد تجعلها خيارًا ممتازًا مساعدًا للمواد الأخرى أو كحل مستقل في حالات الترقيع البسيطة.
كيف تعمل طعوم العظام المجففة بالتجميد (FDBA)؟
تحتفظ طعوم العظام المجففة بالتجميد (FDBA) بمحتواها المعدني، مما يوفر دعامة ناقلة للعظم ممتازة لنمو العظم الجديد. بعد وضعها، تغزو خلايا المريض الطعم تدريجيًا وتستبدله بعظم جديد من خلال عملية تُعرف باسم “الاستبدال الزاحف”.
FDBA يُستخدم عادة في الحفاظ على الحافة السنخية، وتطعيم التجاويف، وتطوير مواقع الزراعة. يندمج بشكل أبطأ من الطعوم الذاتية، لكنه يوفر أساسًا مستقرًا ويمكن التنبؤ به لتجديد العظام. ونظرًا لكونه خاليًا من الخلايا والبروتينات الحية، فلا يحمل أي خطر مناعي ويمكن تخزينه لفترة طويلة، مما يجعله مادة مريحة وشائعة الاستخدام في الممارسة السريرية.
ما هي الطعوم العظمية المجففة والمنزوعة المعادن (DFDBA)؟
يجمع DFDBA بين فوائد التجفيف بالتجميد وإزالة المعادن. تعمل عملية التجفيف بالتجميد على تحسين مدة الصلاحية، بينما تكشف إزالة المعادن عن بروتينات تحفز نمو العظام مثل بروتينات BMP. وهذا يجعل DFDBA ليس فقط موصلًا عظميًا، بل ومحفزًا لتكوين العظام أيضًا، مما يشجع الأنسجة المحيطة على تكوين عظام جديدة.
يُستخدم DFDBA بشكل خاص في تجديد أنسجة اللثة، والعيوب داخل العظام، ورفع الجيوب الأنفية، حيث يكون تحفيز نمو العظام أمرًا بالغ الأهمية. وعلى الرغم من أنه ليس قويًا هيكليًا مثل FDBA، فإن نشاطه البيولوجي يجعله خيارًا مفضلًا في الحالات التي يكون فيها تكوين العظام أهم من الحفاظ على الحجم. وغالبًا ما يُستخدم جنبًا إلى جنب مع مواد طُعم أخرى للحصول على نتائج أفضل.
متى تُستخدم الطعوم القشرية مقابل الإسفنجية؟
الطعوم القشرية كثيفة وتوفر دعمًا هيكليًا ممتازًا، مما يجعلها مثالية لتكبير الحافة، وتطعيم الكتل، والمناطق التي تتطلب قوة ميكانيكية. تندمج بشكل أبطأ لكنها تحافظ على المساحة جيدًا.
أما الطعوم الإسفنجية، فهي مسامية وغنية بمساحات تشبه نقي العظام، مما يعزز الأوعية الدموية وإعادة التشكيل بسرعة أكبر.
وهي مثالية للحفاظ على التجاويف، وعيوب اللثة، أو الطعوم الصغيرة. بعض المنتجات تجمع بين النوعين للاستفادة من ميزتي القوة والشفاء السريع. يعتمد الاختيار على متطلبات موقع الطُعم من حيث الدعم أو الحجم أو سرعة الاندماج.
ما هي فوائد وقيود الطعوم المأخوذة من متبرعين؟
تقدم الطعوم المأخوذة من متبرعين العديد من الفوائد: لا توجد مضاعفات في موقع المانح، وتوافرها مستمر، وتقليل وقت الجراحة. يسهل التعامل معها، ولا تحتاج إلى أخذ عينة من المريض، وتندمج جيدًا في معظم الحالات. وتُعد مفيدة بشكل خاص للمرضى الذين لا يرغبون أو لا يمكنهم الخضوع لجراحة ثانية.
ومع ذلك، تشمل القيود معدل اندماج أبطأ مقارنة بالطعوم الذاتية، وغياب الخلايا المكوّنة للعظام. وعلى الرغم من تعقيمها الكامل، ما زال بعض الأطباء يعبرون عن قلقهم بشأن خطر انتقال الأمراض، وإن كان ضئيلًا جدًا. ومع ذلك، تظل الطعوم المأخوذة من متبرعين خيارًا آمنًا وفعالًا وشائع الاستخدام في العديد من إجراءات زراعة العظام السنية.
لماذا تُلغي الطعوم المأخوذة من متبرعين الحاجة لموقع مانح؟
تُزيل الطعوم المأخوذة من متبرعين تمامًا الحاجة إلى موقع جراحي ثانٍ، مما يعني عدم وجود ألم إضافي أو وقت شفاء أطول أو خطر مضاعفات في موقع المانح. ويُعد ذلك مهمًا بشكل خاص للمرضى الذين يعانون من حالات طبية تبطئ الشفاء أو تزيد من مخاطر الجراحة. وبدون الحاجة لأخذ عظم من جسم المريض، تصبح العملية أسرع وأقل تدخلاً وغالبًا أكثر راحة.
كما أن هذه الميزة تقلل من تكلفة العلاج العامة والانزعاج بعد الجراحة، مما يجعل الطعوم خيارًا جذابًا في الإجراءات السنية البسيطة والمعقدة على حد سواء، خاصة عندما تكون راحة المريض أولوية.
ما العوامل التي تؤثر على معدل اندماج الطعوم المأخوذة من متبرعين؟
تؤثر عدة عوامل على مدى سرعة وفعالية اندماج الطُعم المأخوذ من متبرع. وتشمل هذه صحة المريض، وجودة مادة الطُعم، وكمية الأوعية الدموية في موقع الزرع. يمكن أن تؤخر التدخين أو السكري غير المسيطر عليه أو ضعف نظافة الفم عملية الشفاء. كما أن نوع الطُعم له تأثير، إذ تندمج الطعوم الإسفنجية أسرع من القشرية.
بالإضافة إلى ذلك، فإن استخدام الطعوم مع عوامل نمو أو مع عظم ذاتي يمكن أن يعزز أدائها. كما أن التقنية الجراحية الصحيحة، وتثبيت الطُعم، وتجنب انكشافه من العوامل الحاسمة لنجاح الاندماج. ومع توافر الظروف المناسبة، يمكن للطعوم المأخوذة من متبرعين أن تندمج بشكل متوقع وتدعم النجاح طويل الأمد للترميم.
كيف تقارن الطعوم المأخوذة من متبرعين بالطعوم الذاتية في نسب النجاح؟
بينما تظل الطعوم الذاتية المعيار الذهبي بسبب احتوائها على خلايا حية وعوامل نمو، تقدم الطعوم المأخوذة من متبرعين نسب نجاح مماثلة في العديد من الإجراءات السنية، خاصة عندما تُستخدم في مواقع جيدة التروية. تميل الطعوم الذاتية إلى الاندماج بسرعة أكبر وبشكل أكثر توقعًا، لكنها تتطلب جراحة ثانية.
أما الطعوم المأخوذة من متبرعين، فعلى الرغم من اندماجها الأبطأ قليلًا، فإنها تُزيل الحاجة لموقع مانح وتوفر إطارًا آمنًا وفعالًا لنمو العظام الجديدة. تختلف نسب النجاح حسب نوع الطُعم والحالة السريرية، لكن في العديد من الحالات الروتينية مثل الحفاظ على الحافة أو رفع الجيوب الأنفية، تحقق الطعوم المأخوذة من متبرعين نجاحًا يقارب الطعوم الذاتية عند اختيارها وتطبيقها بشكل صحيح.
ما هي الطعوم الحيوانية وما مدى فعاليتها؟
الطعوم الحيوانية هي مواد زراعة عظمية مشتقة من أنواع غير بشرية، وغالبًا من الأبقار أو الخنازير أو الخيول.
تُعالج هذه الطعوم لإزالة جميع المواد العضوية، لتترك هيكلًا معدنيًا متوافقًا حيويًا يدعم نمو العظام الجديدة. وتُعد الطعوم الحيوانية موصلة عظميًا بشكل أساسي، إذ تعمل كهيكل تنمو داخله عظام المريض الطبيعية.
وهي فعالة جدًا في إجراءات مثل رفع الجيوب الأنفية، والحفاظ على الحافة، وتجديد الأنسجة الداعمة للأسنان. وعلى الرغم من اندماجها الأبطأ من الطعوم الذاتية، فإن استقرارها طويل الأمد ومعدل مضاعفاتها المنخفض يجعلها خيارًا موثوقًا وشائع الاستخدام في طب زراعة العظام السنية.
ما هي المصادر الحيوانية المستخدمة في الطعوم الحيوانية؟
أكثر المصادر الحيوانية استخدامًا للطعوم الحيوانية هي الأبقار والخنازير والخيول. وتتميز عظام هذه الأنواع بتشابهها الكبير مع العظام البشرية من حيث البنية والتركيب.
- عظم الأبقار هو الأكثر استخدامًا بسبب بنيته المسامية وقوته الميكانيكية.
- عظم الخنازير يشبه هيكليًا العظم البشري ويوفر توصيلًا عظميًا جيدًا.
- عظم الخيول معروف بتوافقه وبمعدل امتصاصه البطيء، مما يجعله مناسبًا للدعم طويل الأمد.
تُعالج هذه الطعوم بشكل مكثف لإزالة جميع المواد المسببة للمناعة، مما يضمن سلامتها وتوافقها مع الأنسجة البشرية.
لماذا يُستخدم عظم الأبقار على نطاق واسع في الإجراءات السنية؟
يُستخدم عظم الأبقار على نطاق واسع في زراعة العظام السنية نظرًا لتشابهه الهيكلي مع العظم الإسفنجي البشري. يحتوي على مصفوفة طبيعية مسامية تُسهل التوصيل العظمي، مما يسمح للعظم الجديد بالنمو داخله وحوله.
تخضع الطعوم الحيوانية المشتقة من الأبقار مثل Bio-Oss® لمعالجة دقيقة لإزالة جميع المواد العضوية مع الحفاظ على الإطار المعدني.
ينتج عن ذلك مادة طُعم آمنة ومستقرة ومتوافقة حيويًا بدرجة عالية. وتُفضل بشكل خاص في رفع الجيوب الأنفية والحفاظ على الحافة بفضل بطء امتصاصها، مما يوفر دعمًا طويل الأمد بينما يحل العظم الطبيعي للمريض محلها تدريجيًا.
كيف تُعالج الطعوم الحيوانية المأخوذة من الخنازير للاستخدام السني؟
تُستخلص الطعوم الحيوانية المأخوذة من الخنازير من عظامها وتخضع لمعالجات كيميائية وحرارية وإنزيمية لإزالة البروتينات والدهون والمواد العضوية الأخرى التي قد تسبب تفاعلات مناعية. تحافظ هذه المعالجات على المصفوفة المعدنية الطبيعية للعظم، مما يجعلها هيكلًا موصلًا عظميًا مناسبًا. ويُعقم الطُعم الناتج وغالبًا يُطحن إلى حبيبات أو يُشكل في كتل للاستخدام الجراحي.
الخرافية الخنازية تزداد شيوعًا نظرًا لتوافقها الحيوي وتشابهها البنيوي مع العظم البشري. وهي مفيدة بشكل خاص في إجراءات اللثة وزراعة الأسنان التي تتطلب دعماً متوسطاً واندماجاً موثوقاً.
ما الذي يجعل عظم الحصان مناسبًا للترقيع؟
يتميز عظم الحصان بتوازن فريد بين القوة الميكانيكية والامتصاص البطيء، مما يجعله مناسبًا للحالات التي تتطلب استقرارًا طويل الأمد. يتشابه تركيبه من الكولاجين والمعادن بشكل كبير مع العظم البشري.
من خلال إزالة المستضدات بالإنزيمات والمعالجة الحرارية، يتم التخلص من جميع المكونات المسببة للمناعة، مما يترك هيكلًا متوافقًا حيويًا. وتُقدَّر الطعوم العظمية المشتقة من الحصان بمرونتها في الشكل والبنية، كما أن بعض تقنيات المعالجة تحافظ حتى على الكولاجين الطبيعي، مما يعزز تكوين العظم.
تجعل هذه الخصائص طعوم الحصان فعالة في الإجراءات مثل زيادة حجم العرف الفكي، ورفع الجيب الأنفي، ومعالجة العيوب الكبيرة حيث تكون السلامة الهيكلية أمرًا أساسيًا.
كيف تتم معالجة الطعوم الحيوانية لضمان التوافق الحيوي؟
لضمان التوافق الحيوي، تُخضع الطعوم الحيوانية لمجموعة من المعالجات الميكانيكية والحرارية والكيميائية المصممة لإزالة جميع المواد العضوية والمستضدات. تعمل هذه العمليات على إزالة المكونات الخلوية والبروتينات والعوامل المسببة للأمراض المحتملة مع الحفاظ على المصفوفة المعدنية غير العضوية الضرورية لتجديد العظم.
يُعقَّم الهيكل الناتج ويُختبر ويُشكَّل للاستخدام السريري. وتعد المعالجة الصحيحة ضرورية لتقليل خطر الرفض المناعي أو العدوى أو الالتهاب، مما يضمن اندماج الطعم بأمان مع عظم المريض ويعمل بفعالية كمادة ناقلة لتكوين العظم.
ما هي عملية إزالة البروتينات في الطعوم الحيوانية؟
إزالة البروتينات هي خطوة أساسية في تحضير الطعوم الحيوانية، وتتضمن إزالة جميع البروتينات العضوية التي قد تحفز استجابة مناعية لدى الإنسان. يتم ذلك عادة باستخدام الحرارة أو المذيبات الكيميائية أو المعالجات الإنزيمية. وتحافظ العملية على البنية غير العضوية للعظم مع إزالة بقايا الخلايا والمستضدات.
تحافظ الطعوم منزوعة البروتين، مثل العظم البقري غير العضوي، على بنيتها الطبيعية، مما يجعلها هياكل ممتازة لنقل العظم. من خلال إزالة البروتينات، تضمن العملية أن يكون الطعم خاملًا بيولوجيًا، مما يقلل خطر الرفض ويعزز الأمان والنجاح الطويل الأمد في الإجراءات السنية.
كيف تؤثر المعالجة الحرارية على خصائص الطعوم الحيوانية؟
تُستخدم المعالجة الحرارية (وتسمى أيضًا المعالجة الحرارية الجافة) لتعقيم المواد الطُعمية الحيوانية وإزالة البقايا العضوية، بما في ذلك البروتينات والعوامل الممرضة. تُضبط درجة الحرارة والمدة بعناية لتجنب الإضرار بالبنية المعدنية للطعم.
قد تؤدي المعالجة بدرجات حرارة عالية (أعلى من 600°C) إلى تغييرات في تبلور العظم، مما يجعله أقل قابلية للامتصاص ويقلل من نشاطه الحيوي. وعلى الرغم من أن هذا يزيد من الثبات على المدى الطويل، إلا أنه قد يبطئ الاندماج.
تحافظ المعالجة بدرجات حرارة منخفضة على البنية المسامية للطعم بشكل أفضل وتدعم نمو العظم. تُختار الطريقة المناسبة لتحقيق التوازن بين الأمان والتوافق الحيوي والأداء وفقًا للمؤشر السريري.
ما المعالجات الكيميائية التي تزيل المكونات العضوية؟
تستخدم المعالجات الكيميائية محاليل مثل بيروكسيد الهيدروجين أو الإيثانول أو الأحماض لإذابة وإزالة الدهون والبروتينات والخلايا المتبقية من العظم المشتق من الحيوانات. تزيل هذه المواد الكيميائية المكونات المسببة للمناعة بفعالية دون تغيير كبير في المصفوفة المعدنية للعظم. تتضمن بعض العمليات أيضًا هضمًا إنزيميًا لاستهداف بروتينات محددة مثل الكولاجين.
بعد المعالجة الكيميائية، يُغسل الطعم ويُجفف ويُعقَّم. تُعد هذه الخطوات ضرورية لضمان التعقيم والتوافق الحيوي وسلامة المريض، خصوصًا في الطعوم المستخدمة لتطوير مواقع الزرع أو تجديد اللثة. تضمن المعالجة الكيميائية الصحيحة أن الطعم الحيواني لن يثير استجابة مناعية.
ما مزايا استخدام الطعوم الحيوانية؟
تقدم الطعوم الحيوانية عدة مزايا، فهي متوفرة بسهولة، وفعالة من حيث التكلفة، ومتوافقة حيويًا، مما يجعلها بديلاً ممتازًا عندما لا تكون الطعوم الذاتية أو البشرية ممكنة. توفر الطعوم الحيوانية مصفوفة معدنية طبيعية تدعم نمو العظم والحفاظ على حجمه.
وعلى عكس الطعوم الذاتية، لا تتطلب موقع جراحة ثانٍ، مما يقلل من معاناة المريض. كما أن امتصاصها البطيء يوفر دعماً هيكلياً ممتداً أثناء الشفاء. وبفضل عمليات المعالجة والتعقيم المتقدمة، تعتبر الطعوم الحيوانية الحديثة آمنة وفعالة لمعظم احتياجات ترقيع العظام السنية، بما في ذلك رفع الجيوب الأنفية، وزيادة العرف، وتحضير مواقع الزرع. يجعلها ثباتها وسهولة التنبؤ بنتائجها جزءاً أساسياً من الممارسة السريرية اليومية.
لماذا تتوفر الطعوم الحيوانية بكميات كبيرة؟
تُستخرج الطعوم الحيوانية من حيوانات يتم تربيتها لأغراض طبية أو غذائية، مما يضمن إمداداً ثابتاً وقابلاً للتوسع. وبما أنها لا تعتمد على متبرعين بشريين أو أنسجة من المريض، يمكن إنتاجها بكميات كبيرة ومعالجتها وتخزينها بكفاءة.
وهذا يجعلها ذات قيمة خاصة في العيادات ذات معدلات الجراحة العالية أو في المناطق التي يكون فيها الوصول إلى الطعوم الذاتية محدوداً. تتيح وفرتها للأطباء تقديم إجراءات ترقيع العظم دون تأخير، كما تقلل من الاعتماد على عوامل المريض مثل صحة موقع المتبرع أو كمية العظم المتاحة. تضمن عملية الإنتاج التجارية توفر إمدادات ثابتة وجودة موحدة بين الدفعات.
كيف تحافظ الطعوم الحيوانية على البنية الطبيعية للعظم؟
من خلال المعالجة الدقيقة والحفاظ على البنية، تحتفظ الطعوم الحيوانية ببنيتها التربيقية (الإسفنجية) أو القشرية الأصلية، والتي تشبه العظم البشري بشكل كبير. هذه البنية الطبيعية ضرورية لنقل العظم، إذ تسمح للخلايا العظمية الجديدة بالالتصاق والهجرة والنمو. تدعم البنية المسامية التوعية الدموية وتسهل الاندماج في موقع الزرع.
يساعد الحفاظ على الشكل الهندسي الطبيعي للعظم على تحسين الثبات والحفاظ على الحجم، خاصة في رفع الجيوب الأنفية والحفاظ على العرف الفكي. يستخدم المصنعون إزالة البروتينات بدرجات حرارة منخفضة وتقنيات تنظيف لطيفة للحفاظ على هذه الخصائص، مما يؤدي إلى طعوم تتصرف بشكل مشابه للعظم الطبيعي أثناء الشفاء.
ما الذي يجعل الطعوم الحيوانية فعالة من حيث التكلفة للمرضى؟
تُعتبر الطعوم الحيوانية أكثر توفيراً من الطعوم الذاتية (التي تتطلب جراحة ثانية) وغالبًا أقل تكلفة من الطعوم البشرية (التي تعتمد على متبرعين بشريين ولوائح صارمة). وبما أنها تُنتَج على نطاق واسع ومتاحة بسهولة، فإنها تقلل من تكاليف الإجراءات لكل من الطبيب والمريض.
كما أن عمرها الافتراضي الطويل وسهولة تخزينها يقللان التكاليف الإدارية، بينما يُسهم الاستغناء عن الجراحات الإضافية في تقليل وقت العلاج وفترة التعافي للمريض. تجعل هذه العوامل الطعوم الحيوانية خيارًا اقتصاديًا للأشخاص الذين يحتاجون إلى ترقيع عظمي في الإجراءات السنية الروتينية، بما في ذلك تحضير الزرعات، والحفاظ على التجاويف، وتجديد اللثة.
ما القيود التي يجب أن يعرفها المرضى عن الطعوم الحيوانية؟
على الرغم من أن الطعوم الحيوانية آمنة وشائعة الاستخدام، يجب على المرضى معرفة بعض القيود. فهي ليست مولدة للعظم، أي أنها لا تساهم بخلايا عظمية حية. قد يستغرق اندماجها وقتًا أطول مقارنة بالطعوم الذاتية أو بعض الطعوم البشرية. بعض الطعوم الحيوانية قد تبقى غير ممتصة جزئيًا،
مما قد يؤثر على إعادة التشكيل على المدى الطويل. بالإضافة إلى ذلك، قد يكون لدى بعض المرضى اعتراضات ثقافية أو دينية على استخدام مواد مشتقة من الحيوانات. وعلى الرغم من التعقيم الصارم، لا يزال هناك خطر ضئيل لحدوث استجابة مناعية. يساعد مناقشة هذه المخاوف أثناء تخطيط العلاج على ضمان اتخاذ المرضى قرارات مستنيرة تتماشى مع احتياجاتهم السريرية وقيمهم الشخصية.
كم يستغرق امتصاص الطعوم الحيوانية عادة؟
يحدث امتصاص الطعوم الحيوانية ببطء تدريجي، وغالبًا ما يستغرق من 6 إلى 12 شهرًا أو أكثر، اعتمادًا على المنتج والموقع. وعلى عكس الطعوم الذاتية التي تُعاد تشكيلها بسرعة، تم تصميم الطعوم الحيوانية لتوفير دعم هيكلي طويل الأمد بينما يحل عظم المريض محل الطعم تدريجيًا.
قد تبقى بعض المنتجات، مثل العظم البقري غير العضوي، غير ممتصة جزئيًا لسنوات، لكنها تظل تدعم إعادة تشكيل العظم الصحي ووضع الزرعات.
يُعد هذا الامتصاص البطيء مفيدًا في رفع الجيوب الأنفية أو في التعويضات الكبيرة حيث يكون الحفاظ على المساحة أمرًا بالغ الأهمية. تختلف المدة حسب صحة المريض وتقنية الجراحة ونوع مادة الطعم المستخدمة.
ما هي مواد الطعوم العظمية الاصطناعية؟
الطعوم العظمية الاصطناعية، أو الطعوم غير الحيوية، هي مواد مصنّعة تُستخدم لاستبدال أو تجديد العظام في الإجراءات السنية والعظمية. تم تصميمها لتقليد خصائص العظام الطبيعية، حيث تعمل بشكل أساسي كدعامات ناقلة للعظم (osteoconductive) لتكوين عظم جديد.
تشمل المواد الاصطناعية الشائعة السيراميك الفوسفاتية الكلسية، وهيدروكسي أباتيت، وفوسفات الكالسيوم الثلاثي بيتا (β-TCP)، والزجاج الحيوي النشط. هذه الطعوم خالية تمامًا من الأنسجة البيولوجية، مما يجعلها متوافقة حيويًا وآمنة للغاية.
يُستخدم الطعم الاصطناعي عادةً عندما يفضل المرضى الخيارات غير الحيوية أو لتجنب المخاطر المرتبطة بالطعوم الذاتية أو الطعوم المأخوذة من الإنسان أو الحيوان. وهي متعددة الاستخدامات ومناسبة لمختلف الإجراءات السنية، بما في ذلك تحضير مواقع الزرع.
ما أنواع المواد الاصطناعية المتوفرة؟
تتوفر الطعوم العظمية الاصطناعية في مجموعة متنوعة من التركيبات والأشكال، لكل منها خصائص فريدة مصممة لتلبية احتياجات سريرية مختلفة. الأنواع الأكثر شيوعًا تشمل:
- السيراميك الفوسفاتية الكلسية (هيدروكسي أباتيت وفوسفات الكالسيوم الثلاثي بيتا)
- الزجاج الحيوي النشط
- كبريتات الكالسيوم (تُستخدم أحيانًا مع مواد أخرى)
تتوفر هذه المواد على شكل حبيبات أو معاجين أو كتل أو حتى مواد قابلة للحقن، مما يسمح للأطباء باختيار الشكل الأنسب حسب حجم وموقع العيب العظمي. كل مادة توفر توافقًا حيويًا وقدرة ناقلة للعظم ودرجات مختلفة من القابلية للامتصاص، مما يجعل الطعوم الاصطناعية خيارًا موثوقًا وقابلاً للتخصيص.
كيف تعمل السيراميك الفوسفاتية الكلسية كطعوم؟
تشبه السيراميك الفوسفاتية الكلسية، بما في ذلك هيدروكسي أباتيت (HA) وفوسفات الكالسيوم الثلاثي بيتا (β-TCP)، التركيب المعدني لعظام الإنسان. تعمل هذه المواد كدعامات ناقلة للعظم، حيث توجه نمو العظم الجديد من خلال توفير هيكل يمكن لخلايا بناء العظم أن تهاجر إليه.
اعتمادًا على تركيبها، قد تُمتص ببطء (HA) أو بسرعة أكبر (β-TCP)، مما يسمح للأطباء بمواءمة مدة بقاء الطعم مع معدل شفاء المريض. تُستخدم السيراميك الفوسفاتية الكلسية عادةً في الحفاظ على الحافة السنخية، ورفع الجيب الفكي، وتجديد العظم حول الأسنان، وتُعد بديلاً موثوقًا عندما لا يُفضل استخدام الطعوم الحيوية.
ما هي خصائص طعوم هيدروكسي أباتيت؟
هيدروكسي أباتيت (HA) هو سيراميك بطيء الامتصاص ومتوافق حيويًا يحاكي المكون المعدني للعظم الطبيعي. تدعم بنيته المسامية نمو الأوعية الدموية والتصاق الخلايا، مما يعزز تكوين العظم تدريجيًا. وبسبب بطء تحلله، يعد HA مثاليًا للإجراءات التي تتطلب الحفاظ على الحجم لفترات طويلة، مثل الحفاظ على الحافة السنخية أو علاج العيوب العظمية الكبيرة.
على الرغم من أن HA يفتقر إلى الخصائص المحفزة لتكوين العظم (osteogenic) أو المحفزة لتكوين العظم الجديد (osteoinductive)، إلا أن قدرته العالية على نقل العظم تجعله دعامة موثوقة. يمكن استخدامه بمفرده أو مع مواد أسرع امتصاصًا مثل β-TCP لتحقيق توازن بين القوة وسرعة الشفاء. ثباته وأمانه يجعلان منه خيارًا قيّمًا في الطعوم الاصطناعية.
كيف تعمل مواد فوسفات الكالسيوم الثلاثي بيتا؟
فوسفات الكالسيوم الثلاثي بيتا (β-TCP) هو سيراميك قابل للامتصاص يتحلل بشكل أسرع من هيدروكسي أباتيت، عادة خلال 3 إلى 6 أشهر. ومع تحلله، يُستبدل بعظم جديد، مما يجعله مثاليًا للحالات التي تتطلب تجددًا أسرع.
يساعد β-TCP في نقل العظم ويدعم التصاق الخلايا، كما يطلق أيونات الكالسيوم والفوسفات التي تساهم في عملية إعادة تشكيل العظم. وبفضل معدل امتصاصه المتوقع، يُستخدم β-TCP غالبًا في حفظ تجاويف القلع، وعلاج العيوب اللثوية، وتحضير مواقع الزرع. كما يُمزج في كثير من الأحيان مع أنواع أخرى من الطعوم لتحسين سهولة الاستخدام أو تعزيز الأداء البيولوجي في الإجراءات المعقدة.
ما الذي يجعل الزجاج الحيوي فعالًا في تجديد العظم؟
الزجاج الحيوي النشط هو مادة طعم اصطناعية مكونة من أكاسيد السيليكون والكالسيوم والصوديوم والفوسفور. عند زرعها، تتفاعل مع سوائل الجسم لتشكل طبقة من أباتيت الكربونات الهيدروكسية، والتي تعزز التصاق الخلايا البانية للعظم ونمو العظام.
وعلى عكس السيراميك الأخرى، يتميز الزجاج الحيوي بخصائص مضادة للبكتيريا، مما يقلل من خطر العدوى في موقع الطعم. وهو ناقل للعظم ويُظهر بعض القدرة التحفيزية لتكوين العظم الجديد، مما يجعله فعالًا جدًا في تجديد الأنسجة اللثوية والعظمية، والحفاظ على الحافة السنخية، ومعالجة العيوب حول الزرعات. غالبًا ما يُستخدم في شكل حبيبات أو معاجين، ويمكن مزجه مع الطعوم الذاتية أو المأخوذة من الإنسان لتعزيز نتائج التجديد.
كيف تقارن الطعوم الاصطناعية بالمواد الطبيعية؟
تختلف الطعوم الاصطناعية عن الطعوم الطبيعية في أنها مصنعة هندسيًا وليست مأخوذة من مصادر بشرية أو حيوانية. وهي ناقلة للعظم مثل العديد من المواد الطبيعية، لكنها تفتقر إلى الخلايا البانية للعظم الموجودة في الطعوم الذاتية والبروتينات المحفزة لتكوين العظم الموجودة في بعض الطعوم البشرية أو الحيوانية.
ومع ذلك، فإن الطعوم الاصطناعية تُلغي الحاجة لموقع تبرع وتقلل مخاطر انتقال الأمراض، مما يجعلها أكثر أمانًا في بعض الحالات السريرية. قد يكون معدل اندماجها أبطأ، لكن الابتكارات مثل الزجاج الحيوي أو الطعوم المركبة حسّنت من أدائها. وعلى الرغم من أنها ليست نشطة بيولوجيًا، فإنها توفر نتائج يمكن التنبؤ بها، خاصة عند دمجها مع العظم الطبيعي أو المواد الحيوية.
ما هي الخصائص الناقلة للعظم في الطعوم الاصطناعية؟
توفر الطعوم الاصطناعية دعامة تدعم هجرة الخلايا البانية للعظم والتصاقها ونموها، مما يسمح للعظم الطبيعي بالنمو والاندماج مع المادة واستبدالها تدريجيًا. تُعرف هذه الخاصية باسم النقل العظمي (osteoconduction)، وهي ضرورية في عملية تجديد العظام.
تتميز مواد مثل HA وβ-TCP والزجاج الحيوي بدرجة عالية من المسامية، مما يزيد من مساحة السطح للنشاط الخلوي وتغلغل الأوعية الدموية. وعلى الرغم من أنها لا تحفز تكوين العظم بنفسها (كما تفعل المواد المحفزة للعظم)، إلا أن الطعوم الاصطناعية تكون فعالة وموثوقة عند دمجها مع موقع جراحي غني بالتروية الدموية أو مع معززات بيولوجية مثل PRF (الفيبرين الغني بالصفائح).
ما مدى إمكانية التنبؤ بنتائج الطعوم الاصطناعية؟
عند اختيارها ووضعها بشكل صحيح، تُحقق الطعوم الاصطناعية نتائج يمكن التنبؤ بها بدرجة عالية في الإجراءات السنية. يساهم تجانس تصنيعها، ومعدلات امتصاصها المضبوطة، وتعقيمها في أداء موثوق. تعتمد نسبة النجاح بدرجة كبيرة على اختيار الحالة، وتقنية الجراحة، وخصائص المادة.
على سبيل المثال، يُعد β-TCP ممتازًا للمواقع التي تشفى بسرعة، بينما يناسب HA الحالات التي تتطلب الحفاظ على المساحة لفترة طويلة. وعلى الرغم من أنها لا تملك النشاط البيولوجي للطعوم الذاتية، إلا أن الطعوم الاصطناعية تحقق معدلات نجاح عالية في إجراءات مثل زيادة الحافة السنخية، ورفع الجيوب الفكية، وحفظ التجاويف، خصوصًا عند استخدامها مع مواد ذات أصل ذاتي أو بشري.
ما العوامل التي تؤثر على اندماج الطعوم الاصطناعية؟
توجد عدة عوامل تؤثر على مدى اندماج الطعوم الاصطناعية:
- نوع المادة: يمتص HA ببطء، بينما يمتص β-TCP بسرعة أكبر.
- المسامية وملمس السطح: المسامية العالية تسمح بتغلغل الأوعية الدموية بشكل أفضل.
- موقع الطعم: المواقع ذات التروية الدموية الجيدة تلتئم بسرعة أكبر.
- صحة المريض: التدخين والسكري وسوء نظافة الفم قد يبطئون الاندماج.
- التقنية الجراحية: التعامل السليم، والتثبيت، والتغطية المناسبة (مثل الأغشية) ضرورية.
عند استخدامها بشكل صحيح، تندمج الطعوم الاصطناعية جيدًا وتدعم نتائج مستقرة طويلة الأمد. يساعد فهم هذه العوامل الأطباء على تحسين عملية الشفاء وتعزيز نجاح الطعوم في الإجراءات السنية البسيطة والمعقدة.
ما فوائد اختيار الطعوم الاصطناعية؟
تقدم الطعوم الاصطناعية عدة فوائد مهمة. فهي متوافقة حيويًا وآمنة وخالية من مخاطر نقل الأمراض. وبما أنها مصنّعة، فإنها توفر جودة متسقة وأداء يمكن التنبؤ به عبر جميع الدُفعات.
تلغي الطعوم الاصطناعية الحاجة إلى موقع متبرع، مما يقلل من وقت الجراحة وانزعاج المريض. كما أنها متوفرة بكميات كبيرة ويمكن تهيئتها لتناسب تطبيقات متنوعة، من تطعيم التجاويف إلى تكبير الحواف العظمية الكبيرة.
تُعد مرونتها وتوافرها وسهولة استخدامها أدوات قيمة في زراعة الأسنان الحديثة وجراحة اللثة، خاصة عندما يفضل المرضى أو يحتاجون إلى مواد غير بيولوجية.
لماذا تُزيل الطعوم الاصطناعية مخاطر انتقال الأمراض؟
نظرًا لأن الطعوم الاصطناعية غير بيولوجية ومصنعة يدويًا، فهي لا تحمل أي خطر لنقل الأمراض مثل التهاب الكبد أو فيروس نقص المناعة البشرية أو العدوى المرتبطة بالبريونات. وهذا يجعلها جذابة بشكل خاص للمرضى القلقين بشأن سلامة المواد المشتقة من الإنسان أو الحيوان.
بالإضافة إلى ذلك، يتم تصنيع الطعوم الاصطناعية وفقًا لمعايير تنظيمية صارمة، بما في ذلك التعقيم ومراقبة الجودة، مما يضمن منتجًا معقمًا وموثوقًا. كما أن طبيعتها الاصطناعية تمنع أي مشاكل مناعية، مما يقلل من خطر حدوث مضاعفات. بالنسبة للمرضى والأطباء على حد سواء، توفر هذه الطبقة الإضافية من الأمان راحة البال دون التأثير سلبًا على النتائج السريرية.
كيف توفر المواد الاصطناعية جودة ثابتة؟
يتم إنتاج الطعوم العظمية الاصطناعية في بيئات مخبرية مُحكمة، مما يضمن أحجام جسيمات وتركيبات وخصائص امتصاص قياسية.
يعني هذا الثبات أن الأطباء يمكنهم الاعتماد على معالجة وأداء متوقعين، على عكس الطعوم البيولوجية التي قد تختلف بين المتبرعين. كما يسمح التصنيع بتخصيص المنتجات لتناسب الاحتياجات السريرية، من خلال تعديل المسامية والأشكال (مثل الحبيبات أو المعاجين أو الكتل) أو دمجها بعوامل نمو.
تضمن بروتوكولات مراقبة الجودة، بما في ذلك التعقيم والاختبار الميكانيكي والتحقق من الدُفعات، السلامة والفعالية. وهذا الثبات يُسهّل تخطيط الجراحة ويُحسّن من إمكانية التنبؤ بالنتائج في الحالات السنية الروتينية والمعقدة على حد سواء.
ما الذي يجعل الطعوم الاصطناعية مناسبة للعيوب الكبيرة؟
تُعتبر الطعوم الاصطناعية مثالية للعيوب العظمية الكبيرة نظرًا لتوافرها بكميات كبيرة، وإمكانية تشكيلها حسب الحاجة، ومعدلات امتصاصها القابلة للتحكم. توفر هذه الطعوم استقرارًا ممتازًا في الحجم، خاصة عند استخدام مواد مثل هيدروكسي أباتيت، التي تتحلل ببطء وتحافظ على المساحة مع مرور الوقت.
يمكن أيضًا خلط بعض الطعوم الاصطناعية مع الطعوم الذاتية أو المواد البيولوجية لتحسين أدائها الحيوي في إعادة البناء الواسعة.
بالإضافة إلى ذلك، تسمح الأشكال المتقدمة مثل الطعوم المطبوعة بتقنية ثلاثية الأبعاد أو المركبات القابلة للحقن بالتكيف الدقيق مع شكل العيب. وهذا يجعل المواد الاصطناعية خيارًا عمليًا وفعّالًا للإجراءات الترميمية مثل تكبير الحواف العظمية ورفع الجيوب الأنفية وتجديد العظام حول الزرعات.
كيف تعمل الطعوم العظمية المركبة؟
الطعوم العظمية المركبة هي مزيج من مادتين أو أكثر من مواد الطعوم، مصممة لتحقيق أقصى استفادة من مزايا كل نوع. نظرًا لعدم وجود مادة طعم واحدة مثالية، فإن مزجها يتيح للأطباء تخصيص الخصائص البيولوجية والميكانيكية لتناسب احتياجات المريض. غالبًا ما تجمع هذه الطعوم بين هياكل ناقلة للعظم (مثل الطعوم الحيوانية أو الاصطناعية) ومكونات مكوّنة للعظم أو محفزة له (مثل الطعوم الذاتية أو عوامل النمو). والنتيجة هي طعم أكثر تنوعًا يعزز الشفاء السريع، ويحافظ على حجم العظم بشكل أفضل، ويُحسّن النتائج، خصوصًا في العيوب الصعبة مثل تكبير الحواف الكبيرة أو رفع الجيوب الأنفية أو تجديد العظم حول الزرعات.
ما هي أكثر تركيبات الطعوم العظمية فعالية؟
بعض من أنجح الطعوم المركبة تجمع بين الطعوم الذاتية وكلية المنشأ أو الحيوانية أو الاصطناعية، مما يوازن بين النشاط البيولوجي والدعم الهيكلي. من التركيبات الشائعة:
- الطعم الذاتي + الطعم البشري (Allograft): يعزز التحفيز العظمي ويسرع الشفاء دون الحاجة إلى كميات كبيرة من الطعم الذاتي.
- الطعم الحيواني (Xenograft) + الاصطناعي: يوفر الحفاظ على المساحة على المدى الطويل مع توافق حيوي محسن.
- الطعم البشري (Allograft) + β-TCP: يعزز التحلل التدريجي مع دعم دوران العظم السريع.
يمكن تخصيص هذه التركيبات بناءً على حجم العيب وموقعه وصحة المريض وأهداف الإجراء. المفتاح هو اختيار المواد التي تكمل الوظائف البيولوجية والميكانيكية لبعضها البعض.
كيف تعزز خلطات الطعم الذاتي والبشري عملية الشفاء؟
يؤدي خلط الطعوم الذاتية (التي تحتوي على خلايا عظمية حية) مع الطعوم البشرية (التي تحفز وتدعم تكوين العظم) إلى توليد تآزر قوي لتجديد العظام. تبدأ الطعوم الذاتية عملية الشفاء بتوفير الخلايا الحية وعوامل النمو، بينما تزيد الطعوم البشرية من حجم الطعم دون زيادة أضرار موقع المتبرع. هذا المزيج مفيد بشكل خاص عندما تكون كمية العظم الذاتي محدودة، مثل في رفع الجيوب أو تكبير الحواف. يعمل الطعم البشري كسقالة وموسع بيولوجي، بينما يحفز الطعم الذاتي تكوين العظم الجديد، مما يُسرّع الشفاء ويحسن ثبات الطعم في المراحل المبكرة من الاندماج.
ما الفوائد الناتجة عن الجمع بين الطعوم الحيوانية والاصطناعية؟
يسمح الجمع بين الطعوم الحيوانية (مثل عظم الأبقار أو الخنازير) والمواد الاصطناعية (مثل β-TCP أو الزجاج الحيوي) للأطباء بالاستفادة من استقرار الطعوم الحيوانية طويل الأمد مع الامتصاص المتوقع والنشاط الحيوي للمواد الاصطناعية. توفر الطعوم الحيوانية سقالة ناقلة للعظم ممتازة تحافظ على الحجم مع مرور الوقت، بينما يمكن للمواد الاصطناعية مثل الزجاج الحيوي أن تحفز نشاط الخلايا العظمية وتوفر فوائد مضادة للبكتيريا. هذا المزيج مفيد بشكل خاص في الحفاظ على الحواف وتطوير مواقع الزرعات حيث يكون التجديد التدريجي مطلوبًا. يستفيد المرضى من أمان أكبر، وحفاظ جيد على الحجم، وطعم يدعم كلًا من الشفاء قصير الأمد والاستقرار طويل الأمد.
متى يُوصى باستخدام الطعوم المركبة ثلاثية المكونات؟
تُوصى الطعوم المركبة ثلاثية المكونات في العيوب الكبيرة أو المعقدة التي لا تستطيع مادة واحدة تلبية جميع المتطلبات البيولوجية والهيكلية فيها. مثال شائع هو مزيج الطعم الذاتي + الحيواني + الاصطناعي، حيث:
- الطعم الذاتي يوفر خلايا مكونة للعظم الحي،
- الطعم الحيواني يحافظ على المساحة والبنية، و
- الطعم الاصطناعي يحسن قابلية التعامل أو يضيف نشاطًا بيولوجيًا.
تُعد هذه التركيبات مثالية لإعادة بناء الحواف، ورفع الجيوب الأنفية، أو عيوب العظم حول الزرعات عندما يكون العظم الطبيعي محدودًا. تساعد المقاربة متعددة المواد في تحقيق توازن بين معدلات الامتصاص، والإمكانات التكوينية للعظم، والدعم الميكانيكي، مما يؤدي إلى نتائج أفضل في الحالات الصعبة أو عالية الخطورة.
كيف يتم تحضير وتطبيق الطعوم المركبة؟
يتم عادةً خلط الطعوم المركبة في العيادة باستخدام أدوات أو مجموعات خلط معقمة. يمكن دمج المواد على شكل حبيبات جافة أو ترطيبها بمحلول ملحي أو دم، أو مزجها مع مواد حيوية مثل الفيبرين الغني بالصفائح (PRF) أو شفط نخاع العظم لتعزيز قدرتها التجديدية. بعد الخلط، يتم تشكيل المركب أو تعبئته في موقع العيب وغالبًا يُغطى بغشاء لتوجيه تجديد العظم. يضمن التحضير الصحيح التفاعل الأمثل بين المواد وسهولة التعامل والأداء البيولوجي الممتاز، مما يسمح بملء عظمي أفضل وسلامة هيكلية ونجاح طويل الأمد في زراعة الأسنان وجراحة الفم.
ما هي النسب المثلى لخلطات الطعوم المركبة المختلفة؟
تعتمد النسبة المثلى في الطعوم المركبة على الأهداف السريرية ونوع العيب وسلوك المادة. تشمل الإرشادات الشائعة:
- الطعم الذاتي: الطعم البشري بنسبة 1:1 أو 1:2، لتحقيق توازن بين النشاط التكويني وحجم الطعم.
- الطعم الحيواني: الطعم الاصطناعي بنسبة 3:1 أو 2:1، للحفاظ على قوة السقالة مع تحسين النشاط الحيوي.
- الطعم الثلاثي (ذاتي: حيواني: اصطناعي): غالبًا بنسبة 1:1:1 في العيوب المعقدة.
- يمكن أن يؤدي استخدام كمية كبيرة جدًا من المادة الاصطناعية إلى تأخير الارتشاف، في حين أن كمية قليلة جدًا من الطُعم البنيوي قد تُضعف الحفاظ على الحجم. يقوم الأطباء بتخصيص هذه النسب بناءً على حجم الطُعم، وتوقعات الشفاء، والأهداف الجراحية. يضمن تعديل المزيج أن يدعم الطُعم كلًا من التجدد المبكر واستقرار الحجم على المدى الطويل.
كيف تؤثر تقنية الخلط على أداء الطُعم؟
تُعد تقنية الخلط الصحيحة أمرًا بالغ الأهمية لأداء الطُعوم المركبة. يضمن الخلط المتجانس توزيعًا متساويًا للمواد، مما يمنع ظهور مناطق ضعيفة أو ارتشاف غير متناسق. قد يؤدي الإفراط في الخلط باستخدام المحلول الملحي أو الدم إلى تخفيف عوامل النمو، بينما يؤدي الخلط غير الكافي إلى تكوّن كتل تُعيق تغلغل الخلايا. استخدام المواد البيولوجية الذاتية (مثل PRF أو شفط نخاع العظم) كعامل رابط يُعزز القدرة التجديدية للطُعم ويحسن التعامل معه. بالإضافة إلى ذلك، فإن تحضير المزيج مباشرة قبل وضعه يضمن نضارته ونشاطه الحيوي. الهدف هو طُعم متماسك ومعبأ جيدًا يتكامل بسلاسة مع العظم المحيط، مما يُحسن الاستقرار الميكانيكي والشفاء البيولوجي.
ممتاز، إليك القسم الأخير من مدونتك حول العوامل التي تحدد أفضل نوع من طعوم العظام لكل مريض، مكتوبًا بأسلوب مهني وموجز (حوالي 100 كلمة لكل عنوان فرعي)، ومتسقًا مع أقسامك السابقة.
ما العوامل التي تحدد أفضل نوع من طُعوم العظام لكل مريض؟
يعتمد اختيار الطُعم العظمي المثالي على عدة عوامل سريرية وخاصة بالمريض. وتشمل هذه حجم ونوع عيب العظم، والتاريخ الطبي للمريض، والعمر، والعادات، ونوع الترميم السني المخطط له. بعض الطعوم تناسب العيوب الصغيرة والمحددة، بينما تُستخدم أخرى لإعادة البناء واسعة النطاق أو التي تتحمل الأحمال. بالإضافة إلى ذلك، تؤثر العوامل البيولوجية مثل كثافة العظم، وقدرة الشفاء، وخطر المضاعفات على الاختيار. من خلال تخصيص نوع الطُعم وفقًا لهذه المتغيرات، يتمكن الأطباء من تحسين كفاءة الشفاء وتقليل خطر الفشل ودعم نجاح الزراعة أو الترميم على المدى الطويل.
كيف يؤثر حجم العيب على اختيار الطُعم؟
يُعد حجم العيب أحد أهم العوامل في اختيار الطُعم.
- العيوب الصغيرة، مثل الناتجة عن قلع سن واحد، قد تتطلب فقط مواد ناقلة للعظم مثل الطعوم الصناعية أو الحيوانية.
- العيوب المتوسطة تستفيد من مزيج من الطعوم الناقلة والمحفزة للعظم مثل الطعوم البشرية أو المركبة.
- العيوب الكبيرة تتطلب طعومًا ذات ثبات هيكلي عالٍ، مثل الطعوم الذاتية أو الطعوم الثلاثية المركبة أو الطعوم الصناعية المصممة خصيصًا.
كلما زاد حجم العيب، زادت أهمية اختيار مواد توازن بين الحفاظ على الحجم والنشاط الحيوي والدعم الميكانيكي لضمان تجدد مثالي.
ما المواد الأفضل لمواقع القلع الصغيرة؟
بالنسبة لمواقع القلع الصغيرة، خصوصًا في المناطق الجمالية أو عند التخطيط لزراعة الأسنان، تُعد المواد التي توفر الحفاظ على المساحة وارتشافًا متوقعًا مثالية. تُستخدم الطعوم الحيوانية (مثل العظم البقري) والطعوم الصناعية (مثل β-TCP أو الزجاج الحيوي النشط) بشكل شائع لأنها متوفرة وسهلة التوافق الحيوي ولا تتطلب موقعًا مانحًا. عادةً ما تُغطى هذه المواد بغشاء قابل للامتصاص لتعزيز تجدد العظم الموجه. في المرضى الأصحاء، توفر هذه الطعوم دعمًا كافيًا للحفاظ على الحافة السنخية مع تقليل زمن الجراحة والانزعاج بعد العملية.
ما الأساليب الموصى بها للعيوب الكبيرة في الفك العلوي؟
تتطلب العيوب الكبيرة في الفك العلوي، مثل الناتجة عن الصدمات أو فقدان الأسنان لفترة طويلة، طعومًا ذات قدرة عالية على تكوين العظم وثبات هيكلي قوي. لا تزال الطعوم الذاتية (المأخوذة من مواقع داخل الفم أو خارجه) المعيار الذهبي نظرًا لاحتوائها على خلايا حية وسرعة اندماجها. عندما يكون حجم الطُعم الذاتي غير كافٍ أو يكون الحصاد غير ممكن، يُوصى بالطعوم المركبة مثل مزيج الطُعم الذاتي مع الحيواني والاصطناعي. يوفر هذا المزيج تحفيزًا بيولوجيًا وثباتًا ميكانيكيًا في الوقت نفسه. بالإضافة إلى ذلك، يجب مراعاة تشريح الجيب الفكي ونمط ارتشاف العظم عند اختيار حجم الطُعم وقوة الدعامة ومعدل الارتشاف لإعادة بناء الفك العلوي.
كيف تؤثر العيوب الرأسية مقابل الأفقية على اختيار المواد؟
تُعد العيوب الرأسية أكثر صعوبة في التطعيم وتتطلب مواد تتميز بثبات حجمي ممتاز وارتشاف بطيء، مثل الطعوم الحيوانية أو هيدروكسي أباتيت أو الطعوم الصناعية المصممة خصيصًا. تقاوم هذه المواد الانهيار وتدعم الحفاظ على الارتفاع الرأسي. أما العيوب الأفقية فيمكن علاجها بنجاح باستخدام مواد أسرع في الارتشاف مثل β-TCP أو الطعوم البشرية أو الطعوم المركبة. في كلا الحالتين، غالبًا ما يُستخدم الغشاء الصلب أو نظام التثبيت لحماية الحجم أثناء الشفاء. يتيح فهم اتجاه العيب للأطباء اختيار المواد المناسبة لاستعادة الشكل الأمثل ودعم الزرعة السنية.
ما العوامل الخاصة بالمريض التي تؤثر على اختيار الطُعم العظمي؟
يُظهر كل مريض تحديات فريدة تؤثر على اختيار الطُعم. تشمل هذه العوامل العمر، والحالة الصحية العامة، والأدوية، وعادات التدخين، وجودة العظم، حيث تؤثر على سرعة الشفاء والاندماج وخطر المضاعفات. بالنسبة للمرضى ذوي الحالات الطبية المعقدة، قد تُفضل الطعوم الصناعية نظرًا لانخفاض التباين البيولوجي وعدم وجود خطر انتقال الأمراض. أما في المرضى الشباب الأصحاء، فقد تُسرّع الطعوم الذاتية أو المركبة عملية الشفاء. يساعد التقييم الدقيق لهذه المتغيرات على تقليل المخاطر وضمان أن يدعم الطُعم الأهداف العلاجية القصيرة والطويلة المدى.
كيف يؤثر العمر على شفاء الطُعم العظمي؟
عادةً ما يلتئم المرضى الأصغر سنًا بسرعة أكبر وقد يتحملون الطعوم الذاتية أو المركبة ذات الخصائص التجديدية القوية. يتميز عظمهم بأنه أكثر غزارة في الأوعية الدموية وأكثر نشاطًا بيولوجيًا، مما يجعل الطعوم سريعة الارتشاف (مثل β-TCP أو الطُعم البشري) خيارًا مناسبًا. أما المرضى الأكبر سنًا، فيكون الشفاء أبطأ ودوران العظم أقل، لذا يُفضل لهم استخدام الطعوم الحيوانية أو الاصطناعية ببطء الارتشاف للحفاظ على الحجم على المدى الطويل. بالإضافة إلى ذلك، قد يعاني كبار السن من حالات جهازية تؤثر على الشفاء، مما يتطلب نهجًا جراحيًا أكثر تحفظًا واستخدام طعوم تضمن اندماجًا متوقعًا دون الاعتماد الكبير على حيوية العظم المضيف.
ما الحالات الطبية التي تمنع استخدام أنواع معينة من الطعوم؟
تؤثر بعض الحالات الطبية على مدى أمان أو فعالية أنواع معينة من الطعوم.
- المرضى ضعيفو المناعة أو الذين يتناولون أدوية البايفوسفونات قد لا يتحملون الطعوم الذاتية بسبب ضعف الالتئام أو خطر العدوى.
- مرضى السكري غير المنضبط واضطرابات المناعة الذاتية قد يعانون من تأخر اندماج الطُعم، لذا يُفضل استخدام الطعوم الحيوانية أو الاصطناعية لتقليل الصدمات الجراحية.
- اضطرابات النزف قد تمنع حصاد العظم الذاتي.
- المرضى الذين لديهم تاريخ من سرطان العظام أو العلاج الإشعاعي قد تكون لديهم قدرة محدودة على التجدد، مما يتطلب استخدام طعوم معززة بالنشاط الحيوي مثل تلك المدعمة بـعوامل النمو أو المكونات الخلوية.
يجب دائمًا تقييم المخاطر الجهازية قبل اختيار مادة الطُعم.
كيف تؤثر عادات التدخين على اختيار مادة الطُعم؟
يقلل التدخين من تدفق الدم ويؤخر الشفاء ويزيد من خطر فشل الطُعم. في المدخنين، قد تؤدي الطعوم التي تعتمد على الأوعية الدموية للمضيف، مثل الطعوم الذاتية، أداءً ضعيفًا. وبدلاً من ذلك، يفضل الأطباء غالبًا الطعوم الحيوانية أو الاصطناعية ذات الارتشاف البطيء والثبات الحجمي، لأنها أقل تأثرًا بضعف تروية الأنسجة. يمكن أن يساعد استخدام PRF أو الأغشية الحاجزة في تقليل بعض المخاطر. يُفضل دائمًا تقليل أو الإقلاع عن التدخين قبل وبعد الجراحة، ولكن عندما لا يكون ذلك ممكنًا، يجب اختيار الطعوم التي تتمتع بسلامة هيكلية عالية ومتطلبات بيولوجية منخفضة.
ما الدور الذي تلعبه كثافة العظام في اختيار المادة؟
تؤثر كثافة العظام على مدى اندماج الطعوم العظمية ودعمها لزراعة الأسنان.
- في العظام منخفضة الكثافة (النوع الرابع)، وخاصة في الجزء الخلفي من الفك العلوي، تُفضل الطعوم بطيئة الامتصاص مثل الطعوم الحيوانية (xenografts) أو الهيدروكسي أباتيت (HA) للحفاظ على المساحة لفترة أطول.
- في العظام عالية الكثافة، يمكن استخدام المواد سريعة الامتصاص مثل فوسفات الكالسيوم ثلاثي القاعدة (β-TCP) أو الطعوم البشرية (allografts) لتسريع الاندماج.
كما تؤثر كثافة العظام على استقرار الزرعة، لذا فإن اختيار الطعوم التي تدعم إعادة تشكيل يمكن التنبؤ بها أمر ضروري. غالبًا ما يُستخدم التصوير المقطعي المحوسب (CBCT) لتقييم جودة العظام وتوجيه الطبيب في اختيار مادة الطعم ذات معدل الامتصاص وقدرة التحمل المناسبة.
كيف يؤثر نوع الترميم النهائي المقصود على اختيار الطعم؟
يؤثر نوع الترميم السني النهائي، سواء كان زرعة واحدة أو جسرًا أو طقم أسنان كامل، بشكل كبير على اختيار الطعم. الترميمات التي تتحمل حملاً ميكانيكيًا أكبر أو تتطلب نتائج جمالية عالية تحتاج إلى طعوم تتمتع بثبات حجمي أقوى، أو اندماج أسرع، أو دعم أطول لإعادة التشكيل. على سبيل المثال، قد تتطلب الزرعات الفردية طعومًا سريعة الالتئام للتركيب السريع، بينما تحتاج إعادة بناء الأقواس الكاملة إلى طعوم تحافظ على العرض والارتفاع لفترات طويلة. يجب أخذ موقع الزرعة وتوقيتها ومتطلبات التحمل بالحسبان عند اختيار مادة الطعم.
ما هي المواد الأفضل لمواقع الزرعات الفردية؟
في مواقع الزرعات الفردية، وخاصة في المناطق الجمالية، غالبًا ما يختار الأطباء الطعوم البشرية أو المواد الصناعية أو الحيوانية ذات الامتصاص المتوقع وخصائص الحفاظ على الحافة العظمية. تساعد هذه المواد في الحفاظ على ملامح الأنسجة الرخوة ودعم وضع الزرعة المثالي دون الحاجة إلى موقع جراحي ثانٍ. إذا كان التركيب الفوري مخططًا، يمكن أن تعزز المواد سريعة الامتصاص مثل β-TCP الاندماج السريع. في الحالات المؤجلة، قد يُفضل استخدام الطعوم الحيوانية بسبب استقرار حجمها على المدى الطويل. الهدف هو ضمان وجود كمية كافية من العظم لتحقيق الاستقرار الأولي والوظيفة طويلة الأمد للزرعة.
ما الاعتبارات المتعلقة بإعادة بناء القوس الكامل؟
تتطلب إعادة بناء القوس الكامل تخطيطًا دقيقًا وغالبًا ما تشمل استعادة حجم عظمي كبير. عادةً ما تُستخدم الطعوم المركبة التي تجمع بين الطعوم الذاتية والحيوانية والصناعية لتلبية الاحتياجات البيولوجية والميكانيكية. يجب أن تدعم هذه الطعوم عدة زرعات، وتحافظ على الحجم مع مرور الوقت، وتتحمل الأحمال التعويضية. في بعض الحالات، تكون الطعوم الكتلية، ورفع الجيوب الأنفية، أو تقنيات تجديد العظام الموجهة ضرورية. تُعد المواد بطيئة الامتصاص مثل الطعوم الحيوانية ضرورية للحفاظ على المساحة، بينما تساعد المواد الحيوية أو الطعوم الذاتية في تسريع الالتئام. يؤثر التخطيط التعويضي، بما في ذلك جدول التحميل ونوع التعويض، بشكل كبير على اختيار الطعم واستراتيجية الجراحة.
كيف يؤثر التحميل الفوري مقابل المؤجل على اختيار الطعم؟
إذا كان من المخطط التحميل الفوري للزرعة، تُفضل المواد التي تعزز تكوين العظم بسرعة وتوفر استقرارًا أوليًا قويًا مثل الطعوم الذاتية أو الطعوم البشرية سريعة الامتصاص. تساعد هذه المواد في الاندماج السريع وتقليل الحركة المجهرية في موقع الزرعة. في بروتوكولات التحميل المؤجل، حيث تُوضع الزرعة بعد الشفاء الكامل، يمكن استخدام الطعوم بطيئة الامتصاص مثل الطعوم الحيوانية أو الهيدروكسي أباتيت (HA) للحفاظ على الحجم والشكل أثناء مرحلة الشفاء. يؤثر توقيت التحميل مباشرة على الحاجة إلى نشاط تجديد أسرع أو دعم هيكلي طويل الأمد، مما يجعله عاملاً رئيسيًا في تخطيط الطعوم.
ما أحدث التطورات في تقنيات الطعوم العظمية؟
تركز الابتكارات الحديثة في زراعة العظام على تعزيز النشاط البيولوجي وتحسين خصائص المواد وتخصيص العلاج لكل مريض. تشمل التطورات دمج عوامل النمو والخلايا الجذعية والمواد الحيوية الذكية التي تعزز تكوين العظم بشكل أسرع وأكثر موثوقية. توفر المواد الصناعية الجديدة ذات الأسطح النانوية وتقنيات الطباعة ثلاثية الأبعاد طعومًا مخصصة الشكل تتناسب مع تشريح المريض. بالإضافة إلى ذلك، تجمع تقنيات هندسة الأنسجة بين الخلايا والهياكل الداعمة لإنشاء بدائل عظمية حية. تهدف هذه الاختراقات إلى تقليل وقت الشفاء وتقليل المضاعفات وتحسين نجاح الزرعات على المدى الطويل في الجراحة السنية والعظمية.
كيف تعزز عوامل النمو نتائج الطعوم العظمية؟
تعمل عوامل النمو على تعزيز فعالية الطعوم العظمية بشكل كبير من خلال تحفيز العمليات الخلوية الضرورية للشفاء. فهي تعزز تجنيد الخلايا وتكاثرها وتمايزها، مما يسرع تكوين العظم الجديد واندماجه. تُستخدم عوامل النمو مثل البلازما الغنية بالصفائح الدموية (PRP)، والفيبرين الغني بالصفائح (PRF)، وبروتينات النمو العظمية (BMPs) على نطاق واسع كمكملات في إجراءات الزراعة. فهي تحسن تكوين الأوعية الدموية وتحفز نشاط بانيات العظم، مما يؤدي إلى تجديد أسرع واستقرار أفضل للطعم. إن دمج عوامل النمو في الطعوم يخصص العلاج حسب احتياجات الشفاء الفردية، مما يؤدي إلى نتائج أفضل، خاصة في الحالات الصعبة أو المرضى ذوي الالتئام الضعيف.
ما الدور الذي تلعبه البلازما الغنية بالصفائح الدموية (PRP)؟
البلازما الغنية بالصفائح الدموية (PRP) هي تركيز من الصفائح المأخوذة من دم المريض نفسه، غنية بعوامل النمو مثل PDGF وTGF-β. عند تطبيقها في مواقع الطعوم العظمية، تعزز PRP تكوين الأوعية الدموية وتكاثر الخلايا وتجديد الأنسجة. تعمل كمحفز بيولوجي يسرع الشفاء ويقلل من مضاعفات ما بعد الجراحة مثل العدوى والتورم. غالبًا ما تُدمج PRP مع مواد الطعوم العظمية لتعزيز تكوين العظم، مما يجعلها مفيدة بشكل خاص في المرضى الذين يعانون من بطء الالتئام أو ضعف العظم. كما أن أصلها الذاتي يقلل من التفاعلات المناعية، مما يجعلها إضافة آمنة وفعالة في زراعة العظام السنية.
كيف تحسن بروتينات النمو العظمية (BMPs) من الشفاء؟
تُعد بروتينات النمو العظمية (BMPs) سيتوكينات قوية تحفز مباشرة الخلايا الجذعية الوسيطة على التحول إلى بانيات العظم. أحدثت BMPs، وخاصة BMP-2 وBMP-7، ثورة في زراعة العظام من خلال تعزيز تجديد العظام حتى في الحالات الصعبة مثل العيوب الكبيرة أو المرضى ذوي الحالات المعقدة. غالبًا ما تُدمج هذه البروتينات في مواد الطعوم أو تُستخدم موضعيًا أثناء الجراحة لزيادة حجم العظم وجودته. تقلل BMPs من وقت الشفاء وتحسن اندماج الطعم وقد تقلل الحاجة إلى الطعوم الذاتية. ومع ذلك، يجب استخدامها بحذر بسبب تكلفتها واحتمال حدوث آثار جانبية.
ما فوائد الفيبرين الغني بالصفائح الدموية (PRF)؟
الفيبرين الغني بالصفائح الدموية (PRF) هو جيل ثانٍ من مركزات الصفائح، يشكل شبكة فيبرين غنية بالصفائح والخلايا البيضاء، ويطلق عوامل النمو ببطء مع مرور الوقت. يعزز PRF شفاء الأنسجة الصلبة والرخوة من خلال تحفيز هجرة الخلايا وتكوين الأوعية وتجديد العظم. مقارنة بـ PRP، يوفر PRF إطلاقًا أكثر استدامة لعوامل النمو ويعمل كهيكل طبيعي يدعم التصاق الخلايا. إن سهولة تحضيره وطبيعته الذاتية يجعلان منه خيارًا شائعًا في الجراحة السنية لتحسين نتائج الطعوم، وتقليل الالتهاب، وتسريع إعادة تشكيل الأنسجة، خاصة لدى المرضى ذوي الالتئام الضعيف.
ما المواد الصناعية الجديدة التي يتم تطويرها؟
تتميز المواد الصناعية الجيل الجديد بأسطح نانوية وهياكل خزفية حيوية ومواد مركبة مصممة لمحاكاة العظم الطبيعي بشكل أفضل. تشمل الابتكارات الهيدروكسي أباتيت النانوي والمركبات الزجاجية الحيوية وأسمنتات فوسفات الكالسيوم ذات القوة الميكانيكية المحسنة ومعدلات الامتصاص المنضبطة. تعزز هذه المواد التصاق الخلايا وتمايزها، مما يحسن التوصيل العظمي والاندماج. بالإضافة إلى ذلك، تسمح الهياكل المطبوعة ثلاثية الأبعاد بالتخصيص الدقيق لشكل العيب وتشريح المريض. تدمج بعض المواد الصناعية عوامل مضادة للميكروبات أو ناقلات لعوامل النمو لتقليل خطر العدوى وتحفيز الشفاء، مما يمثل تحولًا نحو مواد طعوم ذكية ومتعددة الوظائف.
كيف تحسن المواد النانوية تكوين العظم؟
تتميز المواد النانوية بأسطح مصممة على المستوى النانوي لتشبه بشكل وثيق المصفوفة خارج الخلية الطبيعية للعظم. تزيد هذه البنية الدقيقة من مساحة السطح وتحسن التصاق الخلايا العظمية وتكاثرها وتمايزها. تعزز المواد مثل الهيدروكسي أباتيت النانوي امتصاص البروتينات والإشارات الخلوية، مما يؤدي إلى تسريع التمعدن ونمو العظم. تؤدي حيويتها العالية إلى اندماج أسرع وأكثر توقعًا للطعم. علاوة على ذلك، يمكن دمج هذه المواد مع عوامل حيوية أو أدوية لتوصيل مستهدف، مما يجعل الطعوم النانوية أداة قوية لتحسين نتائج تجديد العظام في طب الأسنان والعظام.
ما الذي يجعل الطعوم المطبوعة ثلاثية الأبعاد ثورية؟
تتيح الطعوم العظمية المطبوعة ثلاثية الأبعاد تصنيع هياكل داعمة مخصصة للمريض بدقة تتناسب تمامًا مع موقع العيب، مما يحسن الاستقرار ويقلل وقت الجراحة. باستخدام التصوير الرقمي وتقنيات التصميم بمساعدة الحاسوب (CAD)، يمكن طباعة الطعوم بأحجام وأشكال مسامية وخصائص ميكانيكية مخصصة لتحسين تغلغل الخلايا وتكوين الأوعية. كما تتيح هذه التقنية دمج العوامل الحيوية أو عوامل النمو مباشرة أثناء الطباعة. تُحدث الطباعة ثلاثية الأبعاد ثورة في زراعة العظام من خلال توفير طعوم شخصية ومعقدة وقابلة للتكرار، مما يحسن الشفاء والنتائج الوظيفية، خاصة في العيوب الكبيرة أو غير المنتظمة.
كيف تتكيف المواد الحيوية الذكية مع تقدم الشفاء؟
تستجيب المواد الحيوية الذكية بشكل ديناميكي للبيئة البيولوجية، فتُطلق الأيونات أو عوامل النمو أو الأدوية استجابةً لمؤشرات الشفاء مثل تغيّر درجة الحموضة أو الإنزيمات أو الإجهاد الميكانيكي. يمكن لهذه المواد أن تنظم الالتهاب وتحفز تكوين العظم أو تمنع العدوى في مراحل محددة من الشفاء. من خلال تعديل خصائصها بمرور الوقت، تعزز المواد الذكية الاندماج السلس وتقلل المضاعفات مثل رفض الطعم أو العدوى. تمثل هذه الابتكارات خطوة نحو تجديد عظام مخصص ومتحكم به، يضمن أن تعمل المواد بانسجام مع آليات الشفاء الطبيعية للجسم.
ما الأساليب الواعدة في هندسة الأنسجة؟
تجمع هندسة الأنسجة بين الهياكل الداعمة والخلايا والجزيئات المنظمة لإنشاء بدائل عظمية حية. تشمل التقنيات زرع الطعوم بخلايا جذعية أو خلايا مولدة للعظم لتعزيز التجديد. تعمل المفاعلات الحيوية وأنظمة الزراعة ثلاثية الأبعاد على تحسين بقاء الخلايا ووظيفتها قبل الزرع. تهدف هذه الأساليب إلى تجاوز حدود الطعوم التقليدية من خلال توفير أنسجة حية قادرة على إعادة التشكيل وإصلاح العيوب المعقدة. تُظهر التجارب السريرية المبكرة نتائج واعدة في تطبيقات الوجه والفكين، مما يبشر بمستقبل قد تُستبدل فيه الطعوم التقليدية بطعوم هندسية حية.
كيف تُدمج الخلايا الجذعية في الطعوم؟
تُدمج الخلايا الجذعية، وخاصة الخلايا الجذعية الوسيطة (MSCs) المأخوذة من نخاع العظم أو الأنسجة الدهنية، في هياكل الطعوم لتعزيز تكوين العظم. تتحول هذه الخلايا إلى بانيات العظم وتفرز عوامل نمو وتُنظم الاستجابات المناعية، مما يعزز تجديد العظم. يمكن تحضير الطعوم المزروعة بالخلايا الجذعية في العيادة أو في مختبرات متخصصة ودمجها مع مواد حيوية مثل الهلاميات المائية أو السيراميك للزرع. يُظهر هذا النهج إمكانية علاج العيوب الكبيرة أو المعقدة، وتسريع الشفاء، وتحسين توقعات الطعوم، رغم استمرار التحديات التنظيمية والتكلفة لتطبيقها على نطاق واسع.
ما الإمكانيات التي تقدمها تقنيات الهياكل الداعمة (Scaffold)؟
توفر تقنيات الهياكل الداعمة المتقدمة إطارًا لنمو الأنسجة الجديدة، يحاكي البنية المعقدة والخصائص الميكانيكية للعظم. تشمل الابتكارات البوليمرات القابلة للتحلل، والسيراميك المركب، والهلاميات المائية ذات المسامية والقوة القابلة للتعديل. يمكن للهياكل الداعمة توصيل الخلايا أو عوامل النمو أو الأدوية بطريقة منظمة، مما يدعم تجديد العظام خطوة بخطوة. تجعل قدرتها على توجيه تنظيم الأنسجة وتكوين الأوعية منها عنصرًا أساسيًا في معالجة العيوب الكبيرة أو غير المنتظمة. ومع تقدم الأبحاث، تعد تقنيات الهياكل الداعمة بحلول أكثر فعالية وقابلة للتخصيص وأقل تدخلاً، مما يحسن نتائج المرضى ويقلل وقت التعافي.
ما مدى نجاح أنواع الطعوم المختلفة في تركيا؟
تُعد معدلات نجاح الطعوم العظمية في تركيا مماثلة للمعايير العالمية، حيث تُسجل العديد من العيادات نسب بقاء زرعات عالية ورضا مرتفع للمرضى. تمتاز الطعوم الذاتية بأعلى نسب النجاح نظرًا لتوافقها الطبيعي، تليها الطعوم البشرية والحيوانية التي تقدم أداءً جيدًا عند معالجتها بشكل مناسب. كما تُظهر الطعوم الصناعية نتائج واعدة، خاصة عند دمجها بالمواد الحيوية. تستخدم المراكز السنية التركية تقنيات تصوير ومتابعة متقدمة لمراقبة الشفاء والاندماج. عمومًا، تتجاوز نسب النجاح في كثير من الأحيان 90%، مما يعكس جودة الرعاية العالية والخبرة المتوفرة في البلاد.
ما هي معدلات النجاح التي يمكن أن يتوقعها المرضى لكل نوع من أنواع الطعوم؟
في تركيا، تُظهر الطعوم الذاتية معدلات نجاح تصل إلى 95٪ أو أكثر بفضل خصائصها المولدة للعظام. أما الطعوم المأخوذة من متبرعين فعادة ما تحقق نسبة نجاح تتراوح بين 85-90٪، نظرًا لعمليات المعالجة والتعقيم الدقيقة. وتحقق الطعوم الحيوانية معدلات نجاح تبلغ حوالي 85٪، خاصة عند دمجها بعوامل نمو. أما المواد الصناعية فتتفاوت معدلات نجاحها لكنها قد تصل إلى 80-90٪ في الحالات المناسبة. تركز العيادات على الاختيار الدقيق للمرضى والتقنيات الجراحية المثالية لتحسين النتائج. وتتوافق هذه الأرقام مع البيانات الدولية، مما يضمن حصول المرضى على علاج فعال بغض النظر عن نوع الطُعم المستخدم.
كيف تتابع مراكز طب الأسنان في تركيا نتائج الطعوم؟
تستخدم عيادات الأسنان التركية تقنيات رقمية حديثة، بما في ذلك الأشعة المقطعية ثلاثية الأبعاد (CBCT) والتقييمات السريرية، لمراقبة اندماج طعوم العظام. يتم تقييم كثافة العظام وحجمها واستقرار الزرعة في المواعيد الدورية باستخدام الأشعة والفحص السريري. كما تحتفظ العديد من المراكز بسجلات مفصلة للمرضى وتشارك في سجلات وطنية لمتابعة النجاح والمضاعفات على المدى الطويل. يتيح هذا النهج المنهجي للأطباء تخصيص الخطط العلاجية وتحسين البروتوكولات، مما يساهم في تحقيق معدلات نجاح عالية باستمرار في جميع أنحاء البلاد.
ما العوامل التي تساهم في ارتفاع معدلات النجاح في تركيا؟
ترجع معدلات النجاح العالية في تركيا إلى خبرة الجراحين، والتكنولوجيا المتقدمة، والالتزام بالبروتوكولات الدولية. ويساهم استخدام المواد الحديثة إلى جانب العوامل الحيوية مثل PRF في تعزيز الشفاء. كما تساعد توعية المرضى، وضوابط مكافحة العدوى الصارمة، والاختيار الدقيق للحالات في تحسين النتائج. وغالبًا ما تدمج العيادات التركية فرقًا متعددة التخصصات لضمان رعاية شاملة من التشخيص إلى المتابعة. إن التوازن بين جودة الرعاية والتكلفة المعقولة يجذب المرضى ويعزز النتائج المثالية.
لماذا أصبحت تركيا وجهة مفضلة لإجراء طعوم العظام؟
تزداد شهرة تركيا بسبب مزيجها من الأطباء الخبراء، والأسعار المعقولة، والتكنولوجيا المتقدمة. تقدم العديد من العيادات خطط علاج مخصصة في منشآت حديثة مع اعتماد دولي. كما يجعل موقع تركيا الاستراتيجي وبنيتها التحتية السياحية السفر إليها مريحًا، إذ يمكن الجمع بين العلاج والتعافي في بيئة مريحة. بالإضافة إلى ذلك، فإن تركيز تركيا القوي على التعليم والابتكار في طب الأسنان يضمن حصول المرضى على أحدث التقنيات في مجال طعوم العظام.
ما الخبرة التي يتمتع بها أطباء الأسنان في تركيا؟
يتلقى جراحو الأسنان الأتراك تدريبًا دوليًا وغالبًا ما يتقنون جميع تقنيات طعوم العظام، من الطعوم الذاتية إلى الطعوم الصناعية والمعقدة. يحمل العديد منهم تخصصات في جراحة الفم، وأمراض اللثة، وزراعة الأسنان. كما تسهم خبرتهم مع أعداد كبيرة من المرضى في رفع كفاءتهم الجراحية وتسريع العلاج. ويضمن التطوير المهني المستمر إلمامهم بأحدث التقنيات والبروتوكولات، مما يفيد المرضى الباحثين عن حلول متقدمة لتجديد العظام.
كيف تقارن التكاليف في تركيا مع غيرها من الدول؟
تقدم تركيا أسعارًا أقل بكثير لعمليات طعوم العظام وزراعة الأسنان مقارنةً بأوروبا الغربية والولايات المتحدة، حيث تكون عادة أقل بنسبة 40-60٪. ولا تؤثر هذه الأسعار المنخفضة على الجودة، إذ تحافظ العيادات على معايير صارمة وتستخدم مواد معتمدة وموثوقة. وتساعد تكاليف التشغيل المنخفضة والمنافسة القوية على إبقاء الأسعار في متناول المرضى، مما يجذب الزوار الدوليين الباحثين عن القيمة دون التضحية بالنتائج. كما تعزز الشفافية في الأسعار وحزم العلاج المتكاملة من ثقة المرضى ورضاهم.
ما معايير الجودة التي تلتزم بها العيادات التركية؟
تلتزم العديد من عيادات الأسنان التركية بالمعايير الدولية مثل شهادات ISO وتطبيق لوائح الاتحاد الأوروبي للأجهزة الطبية. وغالبًا ما تشارك المرافق في برامج الاعتماد العالمية مثل JCI أو تكون أعضاء في جمعيات طب الأسنان الدولية. تضمن بروتوكولات التعقيم الصارمة، واستخدام المواد المعتمدة من FDA أو CE، والممارسات السريرية المبنية على الأدلة سلامة المرضى. كما تساعد المراجعات المنتظمة وبرامج التعليم المستمر في الحفاظ على مستويات عالية من الرعاية، مما يعزز سمعة تركيا كوجهة موثوقة لعمليات طعوم العظام السنية.
[sc_fs_multi_faq headline-0=”h3″ question-0=”أي نوع من طعوم العظام يلتئم بشكل أسرع؟” answer-0=”الطعوم الذاتية (عظم المريض نفسه) تلتئم عادة بشكل أسرع.” image-0=”” headline-1=”h3″ question-1=”هل هناك مخاطر مرتبطة باستخدام عظم المتبرع؟” answer-1=”نعم، تشمل المخاطر العدوى وردود الفعل المناعية، لكنها نادرة.” image-1=”” headline-2=”h3″ question-2=”كم تبلغ تكلفة كل نوع من طعوم العظام في تركيا؟” answer-2=”تختلف التكاليف بشكل واسع؛ الطعوم الذاتية عادة أغلى بسبب الجراحة، بينما الطعوم المأخوذة من متبرعين أو الصناعية أرخص.” image-2=”” headline-3=”h3″ question-3=”هل يمكن أن تفشل طعوم العظام وما العلامات التحذيرية لذلك؟” answer-3=”نعم، تشمل علامات الفشل الألم والتورم والعدوى وتحرك الطُعم.” image-3=”” headline-4=”h3″ question-4=”كم يجب أن ينتظر المرضى بين الطعم وزراعة السن؟” answer-4=”عادة من 3 إلى 6 أشهر، حسب نوع الطُعم وسرعة الالتئام.” image-4=”” headline-5=”h3″ question-5=”ما العناية بعد العملية المطلوبة لأنواع الطعوم المختلفة؟” answer-5=”تشمل العناية النظافة الفموية، وتجنب الضغط على المنطقة، والالتزام بتعليمات الأدوية.” image-5=”” headline-6=”h3″ question-6=”هل هناك قيود غذائية بعد إجراءات طعوم العظام؟” answer-6=”نعم، يُنصح بتناول الأطعمة الطرية وتجنب الأطعمة الساخنة أو الصلبة لعدة أسابيع.” image-6=”” headline-7=”h3″ question-7=”كيف يمكن للمرضى معرفة ما إذا كان الطعم يلتئم بشكل جيد؟” answer-7=”من خلال غياب الألم أو التورم، والمتابعة بالأشعة وفحوص الطبيب.” image-7=”” headline-8=”h3″ question-8=”ماذا يحدث إذا رفض جسم المريض الطُعم العظمي؟” answer-8=”قد يفشل الطُعم ويتطلب إزالته أو استبداله.” image-8=”” headline-9=”h3″ question-9=”هل يمكن الجمع بين طعوم العظام ورفع الجيوب الأنفية؟” answer-9=”نعم، يتم الجمع بينهما بشكل شائع.” image-9=”” headline-10=”h3″ question-10=”كيف تؤثر أنواع الطعوم المختلفة على الجدول الزمني لزراعة الأسنان؟” answer-10=”الطعوم الذاتية عادة تقصر المدة، بينما الصناعية أو المأخوذة من متبرعين قد تحتاج وقتًا أطول.” image-10=”” headline-11=”h3″ question-11=”ما هي القيود العمرية لأنواع الطعوم المختلفة؟” answer-11=”تُستخدم عادة للبالغين؛ نمو العظام عند الأطفال قد يؤثر على التوقيت.” image-11=”” headline-12=”h3″ question-12=”كيف تؤثر الأدوية على نجاح التئام الطُعم العظمي؟” answer-12=”بعض الأدوية (مثل الستيرويدات أو البايفوسفونات) قد تبطئ الالتئام.” image-12=”” headline-13=”h3″ question-13=”هل يمكن إجراء طعوم العظام تحت تخدير موضعي فقط؟” answer-13=”نعم، غالبًا ما يكون التخدير الموضعي كافيًا.” image-13=”” headline-14=”h3″ question-14=”ما العلامات التي تدل على الحاجة إلى طعم إضافي؟” answer-14=”فقدان العظم المستمر، أو نقص حجم العظم في الصور، أو عدم استقرار الزرعة.” image-14=”” headline-15=”h3″ question-15=”كيف تؤثر أنواع الطعوم المختلفة على النتيجة الجمالية النهائية؟” answer-15=”الطعوم الذاتية عادة تحقق أفضل النتائج الجمالية والوظيفية.” image-15=”” headline-16=”h3″ question-16=”هل يمكن للمرضى اختيار نوع مادة الطعم المفضلة لديهم؟” answer-16=”يمكن للمرضى مناقشة الخيارات، لكن القرار يعتمد على الحالة السريرية.” image-16=”” headline-17=”h3″ question-17=”ما المواعيد اللاحقة المطلوبة بعد عملية الطعم العظمي؟” answer-17=”فحوصات منتظمة لتقييم الالتئام، عادة كل بضعة أسابيع إلى أشهر.” image-17=”” headline-18=”h3″ question-18=”كيف تؤثر المناخات المختلفة على التئام طعوم العظام؟” answer-18=”لا يؤثر المناخ مباشرة، لكنه قد يغير من خطر العدوى وراحة المريض.” image-18=”” headline-19=”h3″ question-19=”ما الحالات الطارئة التي تتطلب تدخلاً فوريًا بعد الطعم؟” answer-19=”الألم الشديد، النزيف المفرط، التورم، الحمى، أو علامات العدوى.” image-19=”” count=”20″ html=”true” css_class=””]